Abstract
Background and Objective
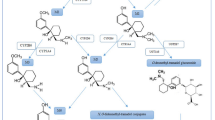
Tramadol hydrochloride is used worldwide as an analgesic drug with a unique dual function. The metabolic enzymes cytochrome P450 (CYP) 3A4, CYP2B6, and CYP2D6 and the various transporters [adenosine triphosphate-binding cassette B1/multidrug resistance 1/P-glycoprotein, organic cation transporter 1, serotonin transporter (SERT), norepinephrine transporter (NET)] and receptor genes (opioid receptor μ 1 gene) give possible genetic differences that might affect the pharmacokinetics and/or pharmacodynamics of tramadol. Therefore, the aim of this review is to present a systematic walkthrough of all possible genetic factors involved in the pharmacology of tramadol.
Method
A systematic literature search was conducted in PubMed and EMBASE involving all metabolic enzymes, drug transporters and receptors, as well as SERT and NET that are involved in the pharmacokinetics and pharmacodynamics of tramadol. An additional search on population pharmacokinetics with genetic factors as covariates was performed separately.
Results
A total of 56 studies (45 cohort and case-control studies, three case reports, six in vitro studies, and two animal studies) were included.
Conclusion
In this systematic review, the current knowledge on all possible genetic factors that might influence the metabolism or clinical efficacy of tramadol has been collected and summarized. Only the effect of CYP2D6 polymorphisms on the metabolism of tramadol and the consequent effect on pain relief has been thoroughly studied and sufficiently established as clinically relevant.


Similar content being viewed by others
References
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879–923.
Kizilbash A, Ngô-Minh CT. Review of extended-release formulations of tramadol for the management of chronic non-cancer pain: focus on marketed formulations. J Pain Res. 2014;7:149–61. doi:10.2147/JPR.S49502.
Pedersen RS, Damkier P, Brosen K. Tramadol as a new probe for cytochrome P450 2D6 phenotyping: a population study. Clin Pharmacol Ther. 2005;77:458–67. doi:10.1016/j.clpt.2005.01.014.
Cicero TJ, Adams EH, Geller A, et al. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22.
Danish Health and Medicines Authority. Summary of product characteristics: tramadol. Danish Health and Medicines Authority. 2015. http://www.produktresume.dk/docushare/dsweb/View/Collection-116.
Sindrup SH, Andersen G, Madsen C, et al. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain. 1999;83:85–90.
Gong L, Stamer UM, Tzvetkov MV, et al. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics 2014.
Bastami S, Haage P, Kronstrand R, et al. Pharmacogenetic aspects of tramadol pharmacokinetics and pharmacodynamics after a single oral dose. Forensic Sci Int. 2014;238:125–32. doi:10.1016/j.forsciint.2014.03.003.
Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:116–21.
Fliegert F, Kurth B, Gohler K. The effects of tramadol on static and dynamic pupillometry in healthy subjects: the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur J Clin Pharmacol. 2005;61:257–66. doi:10.1007/s00228-005-0920-y.
Slanar O, Nobilis M, Kvetina J, et al. Miotic action of tramadol is determined by CYP2D6 genotype. Physiol Res Acad Sci Bohemoslov. 2007;56:129–36.
Matouskova O, Slanar O, Chytil L, Perlik F. Pupillometry in healthy volunteers as a biomarker of tramadol efficacy. J Clin Pharm Ther. 2011;36:513–7. doi:10.1111/j.1365-2710.2010.01203.x.
Stoops WW, Lofwall MR, Nuzzo PA, et al. Pharmacodynamic profile of tramadol in humans: influence of naltrexone pretreatment. Psychopharmacology (Berl). 2012;223:427–38. doi:10.1007/s00213-012-2739-4.
Minami K, Uezono Y, Ueta Y. Pharmacological aspects of the effects of tramadol on G-protein coupled receptors. J Pharmacol Sci. 2007;103:253–60.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi:10.7326/0003-4819-151-4-200908180-00135.
Garcia-Quetglas E, Azanza JR, Sadaba B, et al. Pharmacokinetics of tramadol enantiomers and their respective phase I metabolites in relation to CYP2D6 phenotype. Pharmacol Res Off J Ital Pharmacol Soc. 2007;55:122–30. doi:10.1016/j.phrs.2006.11.003.
Zhou S-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet. 2009;48:689–723. doi:10.2165/11318030-000000000-00000.
Borlak J, Hermann R, Erb K, Thum T. A rapid and simple CYP2D6 genotyping assay: case study with the analgetic tramadol. Metabolism. 2003;52:1439–43.
Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15:218–32.
Gaedigk A, Simon SD, Pearce RE, et al. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234–42. doi:10.1038/sj.clpt.6100406.
Kirchheiner J. CYP2D6 phenotype prediction from genotype: which system is the best? Clin Pharmacol Ther. 2008;83:225–7. doi:10.1038/sj.clpt.6100455.
CYP2D6. In: PharmGKB. http://www.pharmgkb.org/gene/PA128. Accessed 17 Feb 2015.
Human cytochrome P450 (CYP) Allele Nomenclature Committee. http://www.cypalleles.ki.se/. Accessed 19 Jan 2015.
Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig. 1992;70:708–10.
Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312–23.
Nielsen AG, Pedersen RS, Noehr-Jensen L, et al. Two separate dose-dependent effects of paroxetine: mydriasis and inhibition of tramadol’s O-demethylation via CYP2D6. Eur J Clin Pharmacol. 2010;66:655–60. doi:10.1007/s00228-010-0803-8.
Noehr-Jensen L, Zwisler ST, Larsen F, et al. Escitalopram is a weak inhibitor of the CYP2D6-catalyzed O-demethylation of (+)-tramadol but does not reduce the hypoalgesic effect in experimental pain. Clin Pharmacol Ther. 2009;86:626–33. doi:10.1038/clpt.2009.154.
Coller JK, Michalakas JR, James HM, et al. Inhibition of CYP2D6-mediated tramadol O-demethylation in methadone but not buprenorphine maintenance patients. Br J Clin Pharmacol. 2012;74:835–41. doi:10.1111/j.1365-2125.2012.04256.x.
Hedenmalm K, Lindh JD, Sawe J, Rane A. Increased liability of tramadol-warfarin interaction in individuals with mutations in the cytochrome P450 2D6 gene. Eur J Clin Pharmacol. 2004;60:369–72. doi:10.1007/s00228-004-0783-7.
Poulsen L, Arendt-Nielsen L, Brøsen K, Sindrup SH. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther. 1996;60:636–44. doi:10.1016/S0009-9236(96)90211-8.
Pedersen RS, Damkier P, Brøsen K. Enantioselective pharmacokinetics of tramadol in CYP2D6 extensive and poor metabolizers. Eur J Clin Pharmacol. 2006;62:513–21. doi:10.1007/s00228-006-0135-x.
Enggaard TP, Poulsen L, Arendt-Nielsen L, et al. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth Analg. 2006;102:146–50. doi:10.1213/01.ane.0000189613.61910.32.
Stamer UM, Musshoff F, Kobilay M, et al. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther. 2007;82:41–7. doi:10.1038/sj.clpt.6100152.
Halling J, Weihe P, Brosen K. CYP2D6 polymorphism in relation to tramadol metabolism: a study of faroese patients. Ther Drug Monit. 2008;30:271–5. doi:10.1097/FTD.0b013e3181666b2f.
Levo A, Koski A, Ojanpera I, et al. Post-mortem SNP analysis of CYP2D6 gene reveals correlation between genotype and opioid drug (tramadol) metabolite ratios in blood. Forensic Sci Int. 2003;135:9–15.
Paar WD, Poche S, Gerloff J, Dengler HJ. Polymorphic CYP2D6 mediates O-demethylation of the opioid analgesic tramadol. Eur J Clin Pharmacol. 1997;53:235–9.
Stamer UM, Lehnen K, Hothker F, et al. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain. 2003;105:231–8.
Sindrup SH, Madsen C, Brøsen K, Jensen TS. The effect of tramadol in painful polyneuropathy in relation to serum drug and metabolite levels. Clin Pharmacol Ther. 1999;66:636–41. doi:10.1053/cp.1999.v66.103171001.
Stamer UM, Stuber F, Muders T, Musshoff F. Respiratory depression with tramadol in a patient with renal impairment and CYP2D6 gene duplication. Anesth Analg. 2008;107:926–9. doi:10.1213/ane.0b013e31817b796e.
Elkalioubie A, Allorge D, Robriquet L, et al. Near-fatal tramadol cardiotoxicity in a CYP2D6 ultrarapid metabolizer. Eur J Clin Pharmacol. 2011;67:855–8. doi:10.1007/s00228-011-1080-x.
Wang G, Zhang H, He F, Fang X. Effect of the CYP2D6*10 C188T polymorphism on postoperative tramadol analgesia in a Chinese population. Eur J Clin Pharmacol. 2006;62:927–31. doi:10.1007/s00228-006-0191-2.
Shen H, He MM, Liu H, et al. Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17. Drug Metab Dispos Biol Fate Chem. 2007;35:1292–300. doi:10.1124/dmd.107.015354.
Gan SH, Ismail R, Wan Adnan WA, Wan Z. Correlation of tramadol pharmacokinetics and CYP2D6*10 genotype in Malaysian subjects. J Pharm Biomed Anal. 2002;30:189–95.
Li Q, Wang R, Guo Y, et al. Relationship of CYP2D6 genetic polymorphisms and the pharmacokinetics of tramadol in Chinese volunteers. J Clin Pharm Ther. 2010;35:239–47. doi:10.1111/j.1365-2710.2009.01102.x.
Xu J, Zhang X-C, Lv X-Q, et al. Effect of the cytochrome P450 2D6*10 genotype on the pharmacokinetics of tramadol in post-operative patients. Pharm. 2014;69:138–41.
Nasare NV, Banerjee BD, Suryakantrao Deshmukh P, et al. CYP2D6*2 polymorphism as a predictor of failed outpatient tramadol therapy in postherpetic neuralgia patients. Am J Ther. 2013. doi:10.1097/MJT.0b013e31826fc491.
Kirchheiner J, Keulen J-THA, Bauer S, et al. Effects of the CYP2D6 gene duplication on the pharmacokinetics and pharmacodynamics of tramadol. J Clin Psychopharmacol. 2008. doi:10.1097/JCP.0b013e318160f827.
Allegaert K, Rochette A, Veyckemans F. Developmental pharmacology of tramadol during infancy: ontogeny, pharmacogenetics and elimination clearance. Paediatr Anaesth. 2011;21:266–73. doi:10.1111/j.1460-9592.2010.03389.x.
Allegaert K, Anderson BJ, Verbesselt R, et al. Tramadol disposition in the very young: an attempt to assess in vivo cytochrome. Br J Anaesth. 2005;95:231–9. doi:10.1093/bja/aei170.
Allegaert K, Van den Anker JN, Verbesselt R, et al. O-Demethylation of tramadol in the first months of life. Eur J Clin Pharmacol. 2005;61:837–42. doi:10.1007/s00228-005-0045-3.
Allegaert K, van den Anker JN, de Hoon JN, et al. Covariates of tramadol disposition in the first months of life. Br J Anaesth. 2008;100:525–32. doi:10.1093/bja/aen019.
Allegaert K, van Schaik RHN, Vermeersch S, et al. Postmenstrual age and CYP2D6 polymorphisms determine tramadol o-demethylation in critically ill neonates and infants. Pediatr Res. 2008;63:674–9. doi:10.1203/PDR.0b013e31816ff712.
Subrahmanyam V, Renwick AB, Walters DG, et al. Identification of cytochrome P-450 isoforms responsible for cis-tramadol metabolism in human liver microsomes. Drug Metab Dispos Biol Fate Chem. 2001;29:1146–55.
Werk AN, Cascorbi I. Functional gene variants of CYP3A4. Clin Pharmacol Ther. 2014;96:340–8. doi:10.1038/clpt.2014.129.
McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol. 2012;8:371–82. doi:10.1517/17425255.2012.657626.
Hagelberg NM, Saarikoski T, Saari TI, et al. Ticlopidine inhibits both O-demethylation and renal clearance of tramadol, increasing the exposure to it, but itraconazole has no marked effect on the ticlopidine-tramadol interaction. Eur J Clin Pharmacol. 2013;69:867–75. doi:10.1007/s00228-012-1433-0.
Lehtonen P, Sten T, Aitio O, et al. Glucuronidation of racemic O-desmethyltramadol, the active metabolite of tramadol. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2010;41:523–30. doi:10.1016/j.ejps.2010.08.005.
Bhasker CR, McKinnon W, Stone A, et al. Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics. 2000;10:679–85.
Tzvetkov MV, Saadatmand AR, Lötsch J, et al. Genetically polymorphic OCT1: another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin Pharmacol Ther. 2011;90:143–50. doi:10.1038/clpt.2011.56.
Ameyaw MM, Regateiro F, Li T, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–21.
Kanaan M, Daali Y, Dayer P, Desmeules J. Uptake/efflux transport of tramadol enantiomers and O-desmethyl-tramadol: focus on P-glycoprotein. Basic Clin Pharmacol Toxicol. 2009;105:199–206. doi:10.1111/j.1742-7843.2009.00428.x.
Slanar O, Nobilis M, Kvetina J, et al. Pharmacokinetics of tramadol is affected by MDR1 polymorphism C3435T. Eur J Clin Pharmacol. 2007;63:419–21. doi:10.1007/s00228-006-0255-3.
Slanar O, Dupal P, Matouskova O, et al. Tramadol efficacy in patients with postoperative pain in relation to CYP2D6 and MDR1 polymorphisms. Bratisl Lekárske Listy. 2012;113:152–5.
Zhao Q, Sun J, Tao Y, et al. A logistic equation to determine the validity of tramadol from related gene polymorphisms and psychological factors. Pharmacogenomics. 2014;15:487–95. doi:10.2217/pgs.14.22.
Enabah D, El Baz H, Moselhy H. Higher frequency of C.3435 of the ABCB1 gene in patients with tramadol dependence disorder. Am J Drug Alcohol Abuse. 2014;40:317–20. doi:10.3109/00952990.2014.925468.
Fox MA, Jensen CL, Murphy DL. Tramadol and another atypical opioid meperidine have exaggerated serotonin syndrome behavioural effects, but decreased analgesic effects, in genetically deficient serotonin transporter (SERT) mice. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP. 2009;12:1055–65. doi:10.1017/S146114570900011X.
Noskova T, Pivac N, Nedic G, et al. Ethnic differences in the serotonin transporter polymorphism (5-HTTLPR) in several European populations. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1735–9. doi:10.1016/j.pnpbp.2008.07.012.
Ng CH, Easteal S, Tan S, et al. Serotonin transporter polymorphisms and clinical response to sertraline across ethnicities. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:953–7. doi:10.1016/j.pnpbp.2006.02.015.
Rauers NI, Stuber F, Lee E-H, et al. Antagonistic effects of ondansetron and tramadol? A randomized placebo and active drug controlled study. J Pain Off J Am Pain Soc. 2010;11:1274–81. doi:10.1016/j.jpain.2010.03.003.
Zhao X, Huang Y, Ma H, et al. Association between major depressive disorder and the norepinephrine transporter polymorphisms T-182C and G1287A: a meta-analysis. J Affect Disord. 2013;150:23–8. doi:10.1016/j.jad.2013.03.016.
Sagata K, Minami K, Yanagihara N, et al. Tramadol inhibits norepinephrine transporter function at desipramine-binding sites in cultured bovine adrenal medullary cells. Anesth Analg. 2002;94:901–6 (table of contents).
Liu Y-C, Wang W-S. Human mu-opioid receptor gene A118G polymorphism predicts the efficacy of tramadol/acetaminophen combination tablets (ultracet) in oxaliplatin-induced painful neuropathy. Cancer. 2012;118:1718–25. doi:10.1002/cncr.26430.
De Capraris A, Cinnella G, Marolla A, et al. Micro opioid receptor A118G polymorphism and post-operative pain: opioids’ effects on heterozygous patients. Int J Immunopathol Pharmacol. 2011;24:993–1004.
Kim E, Choi C-B, Kang C, Bae S-C. Adverse events in analgesic treatment with tramadol associated with CYP2D6 extensive-metaboliser and OPRM1 high-expression variants. Ann Rheum Dis. 2010;69:1889–90. doi:10.1136/ard.2009.124347.
Allegaert K, Holford N, Anderson BJ, et al. Tramadol and O-desmethyl tramadol clearance maturation and disposition in humans: a pooled pharmacokinetic study. Clin Pharmacokinet. 2014. doi:10.1007/s40262-014-0191-9.
Di Patti F, Fanelli D, Pedersen RS, et al. Modelling the pharmacokinetics of tramadol: on the difference between CYP2D6 extensive and poor metabolizers. J Theor Biol. 2008;254:568–74. doi:10.1016/j.jtbi.2008.06.005.
Acknowledgments
We thank the research librarian Johan Wallin for his support and advice in the literature research.
Conflict of interest
DL, PD and KB report no conflict of interest. No sources of funding were used in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lassen, D., Damkier, P. & Brøsen, K. The Pharmacogenetics of Tramadol. Clin Pharmacokinet 54, 825–836 (2015). https://doi.org/10.1007/s40262-015-0268-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0268-0