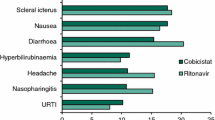
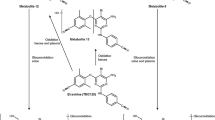
Abstract
Maximal and durable viral load suppression is one of the most important goals of HIV therapy and is directly related to adequate drug exposure. Protease inhibitors (PIs), an important component of the antiretroviral armada, were historically associated with poor oral bioavailability and high pill burden. However, because the PIs are metabolized by cytochrome P450 (CYP) 3A enzymes, intentional inhibition of these enzymes leads to higher drug exposure, lower pill burden, and therefore simplified dosing schedules with this class of drug. This is the basis of pharmacokinetic enhancement. In HIV therapy, two pharmacokinetic enhancers or boosting agents are used: ritonavir and cobicistat. Both agents inhibit CYP3A4, with cobicistat being a more specific CYP inhibitor than ritonavir. Unlike ritonavir, cobicistat does not have antiretroviral activity. Cobicistat has been evaluated in clinical trials and was recently approved in the USA as a fixed-dose combination with the integrase inhibitor, elvitegravir and two nucleos(t)ide analogs. Additional studies are examining cobicistat in fixed-dose combinations with various PIs. In this review, we summarize current knowledge of these agents and clinically relevant drug regimens and ongoing trials. Studies with elvitegravir and the novel PI TMC319011 are also discussed.
Similar content being viewed by others
References
Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [Internet]. AIDSinfo. [cited 2014 May 23]. Available from: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/0.
Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9.
Chen D, Misra A, Garg A. Clinical review 153: lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845–56.
Fantry LE. Protease inhibitor-associated diabetes mellitus: a potential cause of morbidity and mortality. J Acquir Immune Defic Syndr. 2003;32:243–4.
Chiba M, Hensleigh M, Lin JH. Hepatic and intestinal metabolism of indinavir, an HIV protease inhibitor, in rat and human microsomes. Major role of CYP3A. Biochem Pharmacol. 1997;53:1187–95.
Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4 potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25:256–66.
Koudriakova T, Iatsimirskaia E, Utkin I, Gangl E, Vouros P, Storozhuk E, et al. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: mechanism-based inactivation of cytochrome P4503A by ritonavir. Drug Metab Dispos. 1998;26:552–61.
Von Richter O, Burk O, Fromm MF, Thon KP, Eichelbaum M, Kivistö KT. Cytochrome P450 3A4 and P-glycoprotein expression in human small intestinal enterocytes and hepatocytes: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2004;75:172–83.
Klibanov OM. Elvitegravir, an oral HIV integrase inhibitor, for the potential treatment of HIV infection. Curr Opin Investig Drugs. 2009;10:190–200.
Mathias AA, German P, Murray BP, Wei L, Jain A, West S, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010;87:322–9.
Lepist E-I, Phan TK, Roy A, Tong L, Maclennan K, Murray B, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56:5409–13.
Investors: Gilead news release [Internet]. [cited 2013 Dec 4]. Available from: http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArticle_Print&ID=1621754&highlight.
Janssen submits marketing authorisation application to European medicines agency for a fixed-dose combination tablet of HIV-1 medicine darunavir with cobicistat [Internet]. [cited 2013 Dec 4]. Available from: http://www.investor.jnj.com/releasedetail.cfm?releaseid=797389.
Shah BM, Schafer JJ, Priano J, Squires KE. Cobicistat: a new boost for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2013;33:1107–16.
Kempf DJ, Marsh KC, Denissen JF, McDonald E, Vasavanonda S, Flentge CA, et al. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci USA. 1995;92:2484–8.
André P, Groettrup M, Klenerman P, de Giuli R, Booth BL Jr, Cerundolo V, et al. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci USA. 1998;95:13120–4.
Schmidtke G, Holzhütter HG, Bogyo M, Kairies N, Groll M, de Giuli R, et al. How an inhibitor of the HIV-I protease modulates proteasome activity. J Biol Chem. 1999;274:35734–40.
Markowitz M, Saag M, Powderly WG, Hurley AM, Hsu A, Valdes JM, et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–9.
Danner SA, Carr A, Leonard JM, Lehman LM, Gudiol F, Gonzales J, et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. European-Australian Collaborative Ritonavir Study Group. N Engl J Med. 1995;333:1528–33.
Kempf DJ, Marsh KC, Kumar G, Rodrigues AD, Denissen JF, McDonald E, et al. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–60.
NORVIR® (ritonavir) prescribing information [Internet]. [cited 2013 Oct 2]. Available from: http://www.rxabbvie.com/pdf/norvirtab_pi.pdf.
REYATAZ® (atazanavir sulfate) prescribing information [Internet]. [cited 2013 Nov 5]. Available from: http://packageinserts.bms.com/pi/pi_reyataz.pdf.
Clotet B, Bellos N, Molina J-M, Cooper D, Goffard J-C, Lazzarin A, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–78.
PREZISTA® (darunavir) prescribing information [Internet]. 2013 [cited 2013 Sep 4]. Available from: http://www.prezista.com/healthcare/full-prescribing-information.
Orkin C, DeJesus E, Khanlou H, Stoehr A, Supparatpinyo K, Lathouwers E, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013;14:49–59.
Vermeir M, Lachau-Durand S, Mannens G, Cuyckens F, van Hoof B, Raoof A. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab Dispos. 2009;37:809–20.
Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper PJ, Mo HM, et al. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–6.
Sham HL, Kempf DJ, Molla A, Marsh KC, Kumar GN, Chen CM, et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1998;42:3218–24.
Lal R, Hsu A, Chen P, Dennis S, El-Shourbagy T, Locke C, et al. Single dose pharmacokinetics of ABT-378 in combination with ritonavir [abstract I-194]. Toronto: American Society for Microbiology; 1997. p. 279.
Molina J-M, Podsadecki TJ, Johnson MA, Wilkin A, Domingo P, Myers R, et al. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 weeks. AIDS Res Hum Retrovir. 2007;23:1505–14.
Smith CJ, Phillips AN, Youle MS, Sabin CA, Lampe FC, Tsintas R, et al. Treatment outcomes amongst previously antiretroviral-naïve HIV-infected patients starting lopinavir/ritonavir-containing antiretroviral regimens at the Royal Free Hospital. HIV Med. 2007;8:55–63.
KALETRA®(lopinavir/ritonavir) prescribing information [Internet]. Available from: http://www.rxabbvie.com/pdf/kaletratabpi.pdf.
Masquelier B, Breilh D, Neau D, Lawson-Ayayi S, Lavignolle V, Ragnaud J-M, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46:2926–32.
Falcoz C, Jenkins JM, Bye C, Hardman TC, Kenney KB, Studenberg S, et al. Pharmacokinetics of GW433908, a prodrug of amprenavir, in healthy male volunteers. J Clin Pharmacol. 2002;42:887–98.
Furfine ES, Baker CT, Hale MR, Reynolds DJ, Salisbury JA, Searle AD, et al. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother. 2004;48:791–8.
Wire MB, Baker KL, Jones LS, Shelton MJ, Lou Y, Thomas GJ, et al. Ritonavir increases plasma amprenavir (APV) exposure to a similar extent when coadministered with either fosamprenavir or APV. Antimicrob Agents Chemother. 2006;50:1578–80.
LEXIVA (fosamprenavir calcium) prescribing information [Internet]. [cited 2013 Oct 2]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021548s031,022116s015lbl.pdf.
DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43:1–5.
Mathias AA, West S, Hui J, Kearney BP. Dose-response of ritonavir on hepatic CYP3A activity and elvitegravir oral exposure. Clin Pharmacol Ther. 2009;85:64–70.
German P, Warren D, West S, Hui J, Kearney BP. Pharmacokinetics and bioavailability of an integrase and novel pharmacoenhancer-containing single-tablet fixed-dose combination regimen for the treatment of HIV. J Acquir Immune Defic Syndr. 2010;55:323–9.
DeJesus E, Rockstroh JK, Henry K, Molina J-M, Gathe J, Ramanathan S, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379:2429–38.
Rockstroh JK, DeJesus E, Henry K, Molina J-M, Gathe J, Ramanathan S, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62:483–6.
Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–48.
Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63:96–100.
Ramanathan S, Warren D, Wei L, Kearney BP. Pharmacokinetic boosting of atazanavir with the pharmacoenhancer GS-9350 versus ritonavir [poster number A1-1301]. San Francisco, CA USA; 2009. Available from: http://www.hivandhepatitis.com/2009icr/icaac/pdfs/2_Ramanathan.pdf.
Elion R, Cohen C, Gathe J, Shalit P, Hawkins T, Liu HC, et al. Phase 2 study of cobicistat versus ritonavir each with once-daily atazanavir and fixed-dose emtricitabine/tenofovir df in the initial treatment of HIV infection. AIDS. 2011;25:1881–6.
Gallant JE, Koenig E, Andrade-Villanueva J, Chetchotisakd P, DeJesus E, Antunes F, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: week 48 results. J Infect Dis. 2013;208:32–9.
Mathias A, Liu HC, Warren D, Sekar V, Kearney BP. Relative bioavailability and pharmacokinetics of darunavir when boosted with the pharmacoenhancer GS-9350 versus ritonavir [abstract #28]. Sorrento, Italy; 2010. Available from: http://regist2.virology-education.com/11th/docs/16_Mathias.pdf.
Kakuda TN, Opsomer M, Timmers M, Iterbeke K, Van de Casteele T, Hillewaert V, et al. Bioavailablity of two fixed-dose combination formulations of darunavir/cobicistat 800/150 mg compared with darunavir 800 mg and ritonavir 100 mg co-administered as single agents. Barcelona, Spain; 2012. Available from: http://regist2.virology-education.com/2012/13hivpk/docs/37_Kakuda.pdf.
Kakuda TN, Van de Casteele T, Petrovic R, Opsomer M, Tomaka F, Hoetelmans RM. Bioequivalence of darunavir (800 mg) co-administered with cobicistat (150 mg) as either a fixed-dose combination tablet or as single agents under fasted and fed conditions in healthy volunteers [poster MOPE029]. Kuala Lumpur, Malaysia; 2013. Available from: http://pag.ias2013.org/abstracts.aspx?aid=1455.
Ramanathan S, Wang H, Szwarcberg J, Kearney BP. Safety/tolerability, pharmacokinetics, and boosting of twice-daily cobicistat administered alone or in combination with darunavir or tipranavir [abstract P_08]. Barcelona, Spain; 2012. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf.
Dierynck I, Marck HV, Ginderen MV, Jonckers THM, Nalam MNL, Schiffer CA, et al. TMC310911, a novel human immunodeficiency virus type 1 protease inhibitor, shows in vitro an improved resistance profile and higher genetic barrier to resistance compared with current protease inhibitors. Antimicrob Agents Chemother. 2011;55:5723–31.
Hoetelmans RMW, Dierynck I, Smyej I, Meyvisch P, Jacquemyn B, Marien K, et al. Safety and pharmacokinetics of the HIV-1 protease inhibitor TMC310911 coadministered with ritonavir in healthy participants: results from 2 phase 1 studies. J Acquir Immune Defic Syndr. 2014;65:299–305.
Stellbrink H-J, Arastéh K, Schürmann D, Stephan C, Dierynck I, Smyej I, et al. Antiviral activity, pharmacokinetics, and safety of the HIV-1 protease inhibitor TMC310911, coadministered with ritonavir, in treatment-naive HIV-1-infected patients. J Acquir Immune Defic Syndr. 2014;65:283–9.
Hsu A, Granneman GR, Witt G, Locke C, Denissen J, Molla A, et al. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905.
Schmit JC, Ruiz L, Clotet B, Raventos A, Tor J, Leonard J, et al. Resistance-related mutations in the HIV-1 protease gene of patients treated for 1 year with the protease inhibitor ritonavir (ABT-538). AIDS. 1996;10:995–9.
Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52–60.
Burger DM, Huisman A, Van Ewijk N, Neisingh H, Van Uden P, Rongen GA, et al. The effect of atazanavir and atazanavir/ritonavir on UDP-glucuronosyltransferase using lamotrigine as a phenotypic probe. Clin Pharmacol Ther. 2008;84:698–703.
Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59.
Acknowledgments
This manuscript was written with support from grants UO1 AI068632 and UO1 AI068636. Kajal. B. Larson, Kun Wang, Cecile Delille, Igho Otofokun, and Edward P. Acosta have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larson, K.B., Wang, K., Delille, C. et al. Pharmacokinetic Enhancers in HIV Therapeutics. Clin Pharmacokinet 53, 865–872 (2014). https://doi.org/10.1007/s40262-014-0167-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0167-9