Abstract
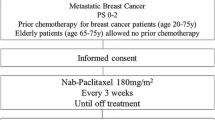
Nanosomal docetaxel lipid suspension (NDLS) [DoceAqualip] is a novel formulation of docetaxel approved in India for the treatment of breast cancer, hormone-refractory prostate cancer, locally advanced squamous cell carcinoma of the head and neck, non-small cell lung cancer and advanced gastric adenocarcinoma. The lipid-based delivery system of NDLS eliminates the need for polysorbate 80 and ethanol, which are contained in the conventional docetaxel formulation and are associated with hypersensitivity reactions and infusion-related toxicities. Because of the diminished potential for NDLS to cause hypersensitivity reactions compared with conventional docetaxel, corticosteroid premedication is not required with NDLS. In a randomized trial in patients with pretreated, locally advanced or metastatic breast cancer, the overall response rate was numerically higher with NDLS than with conventional docetaxel, and post-dose adverse events in the NDLS group were considered manageable and most resolved without sequelae, despite the lack of corticosteroid premedication.

Similar content being viewed by others
References
National Institute for Health and Care Excellence (NICE). Advanced breast cancer diagnosis and treatment. 2014. https://www.nice.org.uk/guidance/cg81/. Accessed 24 Feb 2017.
National Institute for Health and Care Excellence (NICE). Prostate cancer: diagnosis and management. 2014. https://www.nice.org.uk/guidance/cg175/. Accessed 24 Feb 2017.
Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol. 2014;20(14):3905–15.
Al-Sarraf M. Treatment of locally advanced head and neck cancer: historical and critical review. Cancer Control. 2002;9(5):387–99.
National Institute for Health and Care Excellence (NICE). Ovarian cancer (advanced): bevacizumab 7.5 mg/kg in combination with paclitaxel and carboplatin for first-line treatment. 2013. https://www.nice.org.uk/advice/. Accessed 24 Feb 2017.
National Institute for Health and Care Excellence (NICE). Lung cancer: diagnosis and management. 2011. https://www.nice.org.uk/guidance/CG121/. Accessed 24 Feb 2017.
Montero A, Fossella F, Hortobagyi G, et al. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6(4):229–39.
Barbuti AM, Chen ZS. Paclitaxel through the ages of anticancer therapy: exploring its role in chemoresistance and radiation therapy. Cancers. 2015;7(4):2360–71.
Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs. 2005;65(17):2513–31.
McKeage K. Docetaxel: a review of its use for the first-line treatment of advanced castration-resistant prostate cancer. Drugs. 2012;72(11):1559–77.
Blair HA, Deeks ED. Albumin-bound paclitaxel: a review in non-small cell lung cancer. Drugs. 2015;75(17):2017–24.
Ahmad A, Sheikh S, Ali SM, et al. Development of aqueous based formulation of docetaxel: safety and pharmacokinetics in patients with advanced solid tumors. J Nanomed Nanotechnol. 2015;6(3):1000295.
US Food and Drug Administration. Taxotere® (docetaxel) injection concentrate: full prescribing information. 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/. Accessed 1 Sept 2016.
ten Tije AJ, Verweij J, Loos WJ, et al. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42(7):665–85.
Ahmad A, Sheikh S, Taran R, et al. Therapeutic efficacy of a novel nanosomal docetaxel lipid suspension compared with taxotere in locally advanced or metastatic breast cancer patients. Clin Breast Cancer. 2014;14(3):177–81.
Zhang L, Zhang N. How nanotechnology can enhance docetaxel therapy. Int J Nanomed. 2013;8:2927–41.
Intas Pharmaceuticals Ltd. DoceAqualip 20 mg/80 mg nanosomal docetaxel suspension for injection: the first global NDLS. Usage experience in India. Product monograph. Ahmedabad: Intas Pharmaceuticals Ltd; 2014.
Intas Pharmaceuticals Ltd. DoceAqualip: docetaxel lipid suspension for injection 20 mg or 80 mg/vial. Indian package insert. Ahmedabad: Intas Pharmaceuticals Ltd; 2016.
Thiesen J, Kramer I. Physico-chemical stability of docetaxel premix solution and docetaxel infusion solutions in PVC bags and polyolefine containers. Pharm World Sci. 1999;21(3):137–41.
DOCEAQUALIP: Nanosomal lipid docetaxel suspension [Indian data on Metastatic Breast Cancer]. Oral Presentation at 34th ICON Conference of the Indian Cooperative Oncology Network and Molecular Oncology Society. Hyderabad: Intas Pharmaceuticals Ltd., 2016 (Data on file).
Ashraf M, Sajjad R, Khan MA, et al. Efficacy and safety of a novel nanosomal docetaxel lipid suspension (NDLS) as an anti cancer agent—a retrospective study [abstract no. 156P plus poster]. Ann Oncol. 2016;27(Suppl 9).
Naik R, Khan MA. Doceaqualip in a patient with prostate cancer who had an allergic reaction to conventional docetaxel: a case report. Oncol Lett. 2017;6:341–3.
DoceAqualip 20 mg/80 mg nanosomal docetaxel suspension for injection: the first global NDLS. Usage experience in India. Ahmedabad: Intas Pharmaceuticals Ltd., 2016 (Data on file).
Acknowledgements
During the peer review process, the manufacturer of NDLS was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Kate McKeage is a contracted employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: R. B. Shah, GMERS Medical College and Hospital, Gandhinagar, Gujarat, India; R. Sistla, Pharmacology and Toxicology Division, CSIR-Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad, India.
Rights and permissions
About this article
Cite this article
McKeage, K. Nanosomal Docetaxel Lipid Suspension: A Guide to Its Use in Cancer. Clin Drug Investig 37, 405–410 (2017). https://doi.org/10.1007/s40261-017-0510-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-017-0510-7