Abstract
Background and Objectives
No pharmacokinetic data of intravenous ibuprofen were available in a Chinese population and the published information remained inadequate. The present study aimed to investigate the pharmacokinetic properties of intravenous ibuprofen in healthy Chinese volunteers after single- and multiple-dose administration.
Methods
Twelve subjects received single doses of 200, 400, and 800 mg intravenous ibuprofen, respectively, and multiple doses of 400 mg intravenous ibuprofen, four times per day (every 6 h) till the morning of the sixth day in each study period.
Results
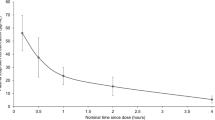
After single doses of 200, 400 and 800 mg and multiple doses of 400 mg intravenous ibuprofen, the main pharmacokinetic parameters obtained were: maximum plasma concentration (C max) 23.05 ± 2.96, 41.90 ± 3.22, 76.06 ± 8.70, and 49.53 ± 3.92 μg/ml, respectively, which were achieved immediately at the end of the infusion; area under the plasma concentration–time curve from time zero to the time of last quantifiable concentration (AUC0–t ) 49.82 ± 10.92, 88.79 ± 12.43, 152.34 ± 25.23, and 106.68 ± 18.94 µg·h/mL, respectively; AUC from time zero to infinity (AUC0−∞) 51.91 ± 10.67, 91.46 ± 12.06, 155.04 ± 25.70, and 108.58 ± 19.49 µg·h/ml, respectively; half-life (t ½) 1.87 ± 0.30, 1.93 ± 0.24, 2.02 ± 0.38, and 1.74 ± 0.26 h, respectively. The accumulation index (AI) was 1.22 ± 0.17 after multiple doses. The most obvious accumulation was observed in males; other parameters revealed no significant differences.
Conclusions
Similar pharmacokinetic properties of intravenous ibuprofen in healthy Chinese volunteers were observed to those reported in a Caucasian population. Multiple doses of intravenous ibuprofen every 6 h caused slight accumulation. Except for the AI, sex did not affect the pharmacokinetics of intravenous ibuprofen.
Chictr.org identifier
ChiCTR-IIR-15007347.



Similar content being viewed by others
References
Beaver WT. Review of the analgesic efficacy of ibuprofen. Int J Clin Prac Suppl. 2003;135:13–7.
Luyk N. Review of pain study of aspirin, ibuprofen and paracetamol. N Z Dent J. 2000;96(424):66.
Bookstaver PB, Miller AD, Rudisill CN, Norris LB. Intravenous ibuprofen: the first injectable product for the treatment of pain and fever. J Pain Res. 2010;3:67–79.
Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34(2):101–54.
Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3–12.
Scott LJ. Intravenous ibuprofen: in adults for pain and fever. Drugs. 2012;72(8):1099–109.
Kroll PB. Intravenous ibuprofen for postoperative pain. Pain Manag. 2012;2(1):47–54.
Caldolor (ibuprofen) injection prescribing information. Nashville: Cumberland Pharmaceuticals Inc.; 2009.
Smith HS, Voss B. Pharmacokinetics of intravenous ibuprofen: implications of time of infusion in the treatment of pain and fever. Drugs. 2012;72(3):327–37.
Morris PE, Promes JT, Guntupalli KK, Wright PE, Arons MM. A multi-center, randomized, double-blind, parallel, placebo-controlled trial to evaluate the efficacy, safety, and pharmacokinetics of intravenous ibuprofen for the treatment of fever in critically ill and non-critically ill adults. Crit Care. 2010;14(3):R125.
Pavliv L, Voss B, Rock A. Pharmacokinetics, safety, and tolerability of a rapid infusion of i.v. ibuprofen in healthy adults. AJHP Off J Am Soc Health-Syst Pharm. 2011;68(1):47–51.
Brocks DR, Mehvar R. Rate and extent of drug accumulation after multiple dosing revisited. Clin Pharmacokinet. 2010;49(7):421–38.
Meineke I, Gleiter CH. Assessment of drug accumulation in the evaluation of pharmacokinetic data. J Clin Pharmacol. 1998;38(8):680–4.
Southworth S, Peters J, Rock A, Pavliv L. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen 400 and 800 mg every 6 h in the management of postoperative pain. Clin Therapeutics. 2009;31(9):1922–35.
Kroll PB, Meadows L, Rock A, Pavliv L. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen (i.v.-ibuprofen) in the management of postoperative pain following abdominal hysterectomy. Pain Prac Off J World Inst Pain. 2011;11(1):23–32.
Singla N, Rock A, Pavliv L. A multi-center, randomized, double-blind placebo-controlled trial of intravenous-ibuprofen (IV-ibuprofen) for treatment of pain in post-operative orthopedic adult patients. Pain Med. 2010;11(8):1284–93.
Krudsood S, Tangpukdee N, Wilairatana P, et al. Intravenous ibuprofen (IV-ibuprofen) controls fever effectively in adults with acute uncomplicated Plasmodium falciparum malaria but prolongs parasitemia. Am J Trop Med Hyg. 2010;83(1):51–5.
Bergese SD, Candiotti K, Ayad SS, Soghomonyan S, Gan TJ, Intravenous Ibuprofen Surveillance Trial Investigational S. The shortened infusion time of intravenous ibuprofen part 1: a multicenter, open-label, surveillance trial to evaluate safety and efficacy. Clin Therapeutics. 2015;37(2):360–7.
Gan TJ, Candiotti K, Turan A, et al. The shortened infusion time of intravenous ibuprofen, part 2: a multicenter, open-label, surgical surveillance trial to evaluate safety. Clin Therapeutics. 2015;37(2):368–75.
Garcia-Martin E, Martinez C, Tabares B, Frias J, Agundez JA. Interindividual variability in ibuprofen pharmacokinetics is related to interaction of cytochrome P450 2C8 and 2C9 amino acid polymorphisms. Clin Pharmacol Ther. 2004;76(2):119–27.
Yoon YR, Shon JH, Kim MK, et al. Frequency of cytochrome P450 2C9 mutant alleles in a Korean population. Br J Clin Pharmacol. 2001;51(3):277–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was sponsored and funded by Beijing Alica Pharmaceutical Sci-Tech Co. Ltd. (Beijing, People’s Republic of China).
Conflict of interest
Yali Shen, Feng Nan, Mei Li, Maozhi Liang, Ying Wang, Zhihui Chen, and Zhu Luo declare that they have no financial relationships with any organizations that might have an interest in the submitted work, nor any other relationships or activities that could influence the submitted work.
Ethical approval
The study protocol was approved by the Independent Ethics Committee of West China Hospital, Sichuan University (Chengdu, China). Written informed consent was obtained from each subject before screening procedures. All procedures in this study were carried out in accordance with the Helsinki declaration.
Rights and permissions
About this article
Cite this article
Shen, Y., Nan, F., Li, M. et al. Pharmacokinetic Properties of Intravenous Ibuprofen in Healthy Chinese Volunteers. Clin Drug Investig 36, 1051–1058 (2016). https://doi.org/10.1007/s40261-016-0453-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0453-4