Abstract
Background and Objective
In Italy, the Italian Pharmaceutical Agency (AIFA) criteria used F3–F4 fibrosis stages as the threshold to prioritise the treatment with interferon (IFN)-free regimens, while in genotype 1 chronic hepatitis C (G1 CHC) patients with fibrosis of liver stage 2, an approach with pegylated interferon (PEG-IFN)-based triple therapy with simeprevir was suggested. The key clinical question is whether, in an era of financial constraints, the application of a universal IFN-free strategy in naïve G1 CHC patients is feasible within a short time horizon. The aim of this study is to perform an economic analysis to estimate the cost-utility of the early innovative therapy in Italy for managing hepatitis C virus (HCV)-infected patients.
Methods
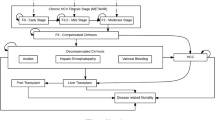
The incremental cost-utility analysis was carried out to quantify the benefits of the early treatment approach in HCV subjects. A Markov simulation model including direct and indirect costs and health outcomes was developed from an Italian National Healthcare Service and societal perspective. A total of 5000 Monte Carlo simulations were performed on two distinct scenarios: standard of care (SoC) which includes 14,000 genotype 1 patients in Italy treated with innovative interferon-free regimens in the fibrosis of liver stages 3 and 4 (F3–F4) versus early-treatment scenario (ETS) where 2000 patients were additionally treated with simeprevir plus PEG-IFN and ribavirin in the fibrosis stage 2 (F2) (based on Italian Medicines Agency AIFA reimbursement criteria). A systematic literature review was carried out to identify epidemiological and economic data, which were subsequently used to inform the model. Furthermore, a one-way probabilistic sensitivity was performed to measure the relationship between the main parameters of the model and the cost-utility results.
Results
The model shows that, in terms of incremental cost-effectiveness ratio (ICER) per quality adjusted life year (QALY) gained, ETS appeared to be the most cost-utility option compared with both perspective societal (ICER = EUR11,396) and NHS (ICER = EUR14,733) over a time period of 10 years. The cost-utility of ETS is more sustainable as it extends the time period analysis [ICER = EUR 6778 per QALY to 20 years and EUR4474 per QALY to 30 years]. From the societal perspective, the ETS represents the dominant option at a time horizon of 30 years. If we consider the sub-group population of treated patients [16,000 patients of which 2000 not treated in the SoC, the ETS scenario was dominant after only 5 years and the cost-utility at 2 years of simulation. The one-way sensitivity analysis on the main variables confirmed the robustness of the model for the early-treatment approach.
Conclusion
Our model represents a tool for policy makers and health-care professionals, and provided information on the cost-utility of the early-treatment approach in HCV-infected patients in Italy. Starting innovative treatment regimens earlier keeps HCV-infected patients in better health and reduces the incidence of HCV-related events; generating a gain both in terms of health of the patients and correct resource allocation.





Similar content being viewed by others
References
Global Burden Of Hepatitis CWG. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–9.
European Centre for Disease Prevention and Control. Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. 2010.
Camma C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–42.
Bruno S, Crosignani A, Facciotto C, et al. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069–76.
Singal AG, Volk ML, Jensen D, et al. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8, 88 e1.
Camma C, Giunta M, Andreone P, et al. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001;34:593–602.
Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87.
Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–26.
Zeuzem S, Berg T, Gane E, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146(430–41):e6.
Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98.
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93.
Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92.
Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–82.
Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603.
Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–14.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–21.
Everson GT, Sims KD, Rodriguez-Torres M, et al. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146:420–9.
Jacobson J, RM. G, Rodriguez-Torres. M, et al. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naive and prior null responder patients: the COSMOS study. In: 48th Annual Meeting of the European Association for the Study of the Liver, Amsterdam, The Netherlands, April 24–28, 2013.
Russo P. La Valutazione Framacoeconomica nel contesto regolatorio Italiano. Pharmacoeconomics—Italian Research Articles. 2008;10:59–75.
Associazione Italiana Economia Sanitaria (AIES). Proposta di Linee-Guida per la valutazione economica degli interventi sanitari. Poilitiche Sanitarie. 2009;10:91–99.
Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–3. Value Health. 2012;15:812–20.
Mennini FS, Marcellusi A, Viti R, et al. Disponibilità a pagare e innovazione: il caso dei farmaci anti-HCV nel Sistema Sanitario Italiano. Glob Reg Health Technol Assess. 2015;2:69–77.
Loreta Kondili LF, Mallano Alessandra, Quaranta Maria Giovanna, Mirra Marco, Liliana Elena Weimer LF, Di Gregorio Massimiliano, Lucattini Stefano, Massella Maurizio, Roberta Terlizzi EO, Magnani Federica, Mattei Alessandra, Rosato Stefano, et al. LA PIATTAFORMA ITALIANA PER LO STUDIO della terapia DELLE EPATITI VIRALI (PITER): il primo grande studio nazionale sull’infezione cronica da virus dell’epatite C. Notiziario dell’Istituto Superiore di Sanità. 2015;28:3–10.
Mennini FS, Marcellusi A, Andreoni M, et al. Health policy model: long-term predictive results associated with the management of hepatitis C virus-induced diseases in Italy. ClinicoEcon Outcomes Res. 2014;6:303–10.
European Centre for Disease Prevention and Control. Annual Epidemiological Report 2013. Reporting on 2011 surveillance data and 2012 epidemic intelligence data. Stockholm: ECDC; 2013. p. 2013.
Agenzia Italiana del Farmaco (AIFA). Modello Algoritmo Terapia HCV. 2015.
Manns MP, Fried MW, Zeuzem S, et al. Simeprevir with peginterferon/ribavirin for treatment of chronic hepatitis C virus genotype 1 infection: pooled safety analysis from Phase IIb and III studies. J Viral Hepatitis. 2015;22:366–75.
D’Offizi G, Cammà C, Schlag M, et al. Optimisation of simeprevir triple therapy: a multivariate logistic regression model using baseline predictors. In: The 28th International Conference on Antiviral Research (ICAR), Rome, Italy, 11–15 May 2015.
DAKLINZA approval price in Italy: Gazzetta Ufficiale della Repubblica Italiana, April 20, 2015.
SOVALDI approval price in Italy: Gazzetta Ufficiale della Repubblica Italiana, November 12, 2014.
EXVIERA approval price in Italy: Gazzetta Ufficiale della Repubblica Italiana, May 18, 2015.
HARVONI approval price in Italy: Gazzetta Ufficiale della Repubblica Italiana, May 5, 2015.
OLYSIO approval price in Italy: Gazzetta Ufficiale della Repubblica Italiana, November 17, 2015.
Marcellusi A, Viti R, Capone A, et al. The economic burden of HCV-induced diseases in Italy. A probabilistic cost of illness model. Eur Rev Med Pharmacol Sci. 2015;19:1610–20.
Petta S, Cabibbo G, Enea M, et al. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59:1692–705.
Cortesi PA, Ciaccio A, Rota M, et al. Management of treatment-naive chronic hepatitis C genotype 1 patients: a cost-effectiveness analysis of treatment options. J Viral Hepatitis. 2015;22:175–83.
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York; 2007.
Giannini EG, Cucchetti A, Erroi V, et al. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World J Gastroenterol. 2013;19:8808–21.
Istituto Nazionale di Statistica (ISTAT). Tavole di mortalità della popolazione italiana. Ripartizione: Italia Anno: 2013. Available: http://demo.istat.it/unitav2012/index.html?lingua=ita. Accessed 25 May 2016.
Craxi L, Camma C, Craxi A. HCV: the best cure possible or the best possible cure? J Viral Hepatitis. 2015;22:627–9.
Negro F, Forton D, Craxi A, et al. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Funding
This analysis was funded by Janssen-Cilag SpA. The authors confirm that the paper is an accurate representation of the study results. FD is an employee of the sponsor and was involved in the study design, data collection and analysis, interpretation of data.
Conflict of interest
FD is employee of Janssen-Cilag SpA.
GT, CC has received consulting fees from Janssen-Cilag SpA, MSD.
AM, RV and FSM declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Marcellusi, A., Viti, R., Damele, F. et al. Early Treatment in HCV: Is it a Cost-Utility Option from the Italian Perspective?. Clin Drug Investig 36, 661–672 (2016). https://doi.org/10.1007/s40261-016-0414-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0414-y