Abstract
Background and Objectives
Secondary hyperparathyroidism is a common consequence of chronic kidney disease. Cinacalcet (Sensipar®) is often prescribed in combination to reduce elevated levels of parathyroid hormone, calcium and phosphorus. The objective of this study was to assess the effects of concomitantly administered therapies of calcium carbonate (CaCO3; TUMS®), sevelamer hydrochloride (HCl; Renagel®) and pantoprazole sodium (Protonix®) on the pharmacokinetics and safety of cinacalcet in healthy subjects.
Methods
Three randomized, open-label, two-way crossover pharmacokinetic studies were conducted in healthy subjects. Participants received single doses of cinacalcet alone or in combination with either CaCO3, sevelamer HCl or pantoprazole. The pharmacokinetic profile of cinacalcet was characterized. Safety assessments including adverse event reporting, changes in vital signs and clinical laboratory measurements were conducted throughout the studies.
Results
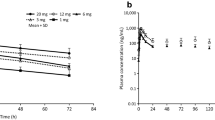
The 90 % confidence intervals for the area under the concentration–time curve from time zero to the last quantifiable concentration (AUClast), area under the concentration–time curve from time zero to infinity (AUC0–∞) and maximum plasma concentration (C max) of cinacalcet were within the accepted range of 80–125 % for both CaCO3 and sevelamer HCl co-administration with cinacalcet. No severe or serious adverse events or clinically relevant changes in physical or laboratory findings occurred during the studies.
Conclusion
The pharmacokinetic parameters of cinacalcet were not affected by co-administration of CaCO3, sevelamer HCl or pantoprazole. Co-administration of these agents with cinacalcet does not require an adjustment of the dose of cinacalcet.



Similar content being viewed by others
References
Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int. 1999;73(Suppl):S14–9.
Eknoyan G, Levin A, Levin NW. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):1–201.
Goodman WG. Recent developments in the management of secondary hyperparathyroidism. Kidney Int. 2001;59(3):1187–201.
Hammerland LG, Garrett JE, Hung BC, Levinthal C, Nemeth EF. Allosteric activation of the Ca2+ receptor expressed in Xenopus laevis oocytes by NPS 467 or NPS 568. Mol Pharmacol. 1998;53(6):1083–8.
Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95(7):4040–5.
Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, et al. The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13(4):1017–24.
Lindberg JS, Moe SM, Goodman WG, Coburn JW, Sprague SM, Liu W, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium × phosphorus in secondary hyperparathyroidism. Kidney Int. 2003;63(1):248–54.
Quarles LD, Sherrard DJ, Adler S, Rosansky SJ, McCary LC, Liu W, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol. 2003;14(3):575–83.
de Francisco ALM, Suranyi M, Cunningham J, Messa P, Locatelli F, Evenepoel P, et al. Oral cinacalcet HCl (AMG 073) for the treatment of hemodialysis patients with secondary hyperparathyroidism (HPT): results of a European/Australian phase 3 study. J Am Soc Nephrol. 2003;14(Suppl):461A (abstract).
Block GA, Goodman WG. Cinacalcet HCl for the treatment of secondary hyperparathyroidism in hemodialysis patients. N Engl J Med. 2004;350:1516–25.
Moe SM, Couburn JW, Quarles LD, et al. Achievement of proposed NKF-K/DOQI bone metabolism and disease targets: treatment with cinacalcet HCl in dialysis patients with uncontrolled secondary hyperparathyroidism (HPT). J Am Soc Nephrol. 2003;14(Suppl):48A (abstract).
Strid H, Simrén M, Björnsson ES. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol Dial Transplant. 2003;18(3):570–5.
Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther. 1999;13(Suppl 3):18–26.
Negro RD. Pharmacokinetic drug interactions with anti-ulcer drugs. Clin Pharmacokinet. 1998;35(2):135–50.
Maton PN, Burton ME. Antacids revisited: a review of their clinical pharmacology and recommended therapeutic use. Drugs. 1999;57(6):855–70.
ICH harmonised tripartite guideline: guideline for good clinical practice. 8. Essential documents for the conduct of a clinical trial. J Postgrad Med. 2001;47(4):264–7.
Feldman M. Comparison of the effects of over-the-counter famotidine and calcium carbonate antacid on postprandial gastric acid. A randomized controlled trial. JAMA. 1996;275(18):1428–31.
Lin MS, Sun P, Yu HY. Evaluation of buffering capacity and acid neutralizing-pH time profile of antacids. J Formos Med Assoc. 1998;97(10):704–10.
Serra AL, Braun SC, Starke A, Savoca R, Hersberger M, Russmann S, et al. Pharmacokinetics and pharmacodynamics of cinacalcet in patients with hyperparathyroidism after renal transplantation. Am J Transplant. 2008;8(4):803–10.
FDA, Guidance for industry—in vivo drug metabolism/drug interaction studies—stud design, data analysis, and recommendations for dosing and labeling. November 1999, National Press Office: Rockville. http://www.fda.gov/ohrms/dockets/98fr/981001gd.pdf. Accessed 5 May 2014.
Bays HE, Dujovne CA. Drug interactions of lipid-altering drugs. Drug Saf. 1998;19(5):355–71.
Sevelamer HCl (Renagel) prescribing information, available at http://www.renagel.com/docs/renagel_pi.pdf. Accessed 12 Jan 2007.
Acknowledgments
These studies were supported by Amgen Inc. The CaCO3 study was conducted by the MDS Harris Clinical Laboratory, Lincoln, NE, USA. The sevelamer HCl study was conducted by DaVita Clinical Research, Minneapolis, MS, USA, with analysis of pharmacokinetic and clinical samples by MDS Pharma Services, Lincoln, NE, USA, and Medtox Laboratories, Inc., Saint Paul, MN, USA, respectively, and data management provided by Clinimetrics, Inc. The pantoprazole study was conducted by MDS Pharma Services with data management provided by Clinimetrics, Inc. Statistical analysis was conducted by Amgen Inc. We thank Helen Gauton and Tom Westgate of Gardiner-Caldwell for providing editing support funded by Amgen. Holly Tomlin (employee and stockholder, Amgen Inc.) was responsible for editing and formatting the manuscript for the journal).
Conflict of Interest Statement
Desmond Padhi, Robert Harris and John T. Sullivan are employees and stockholders of Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padhi, D., Harris, R. & Sullivan, J.T. Effects of Calcium Carbonate, Sevelamer Hydrochloride or Pantoprazole on the Pharmacokinetics of Cinacalcet. Clin Drug Investig 34, 537–544 (2014). https://doi.org/10.1007/s40261-014-0206-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0206-1