Abstract
Purpose of Review
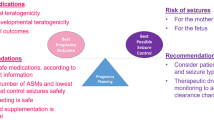
Care of people with epilepsy and gestational capacity throughout pregnancy involves weighing risks of seizures with the known risks of anti-seizure medications, and requires planning and patient counseling.
Recent Findings
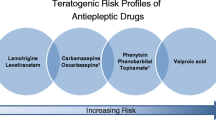
Anti-seizure medications are associated with increased risk of congenital malformations. Further study is needed to understand the risk profile of newer anti-seizure medications in pregnancy. Cognitive outcomes of infants exposed to anti-seizure medications in utero vary, but valproate is associated with an increased risk of cognitive teratogenesis. Topiramate may also have an adverse neurodevelopmental effect though data are conflicting. Anti-seizure medication concentrations fall during pregnancy, so therapeutic drug monitoring and dose adjustment are needed during pregnancy. Peri-conceptual folic acid supplementation has benefits, but the best dose is not known. Anti-seizure medications get into breastmilk, though infant levels are lower than maternal levels and breastfeeding in recommended.
Summary
Recent research highlights that anti-seizure medications have both structural and cognitive teratogenic potential. Further studies into newer anti-seizure medications as well as folic acid dosing are needed to optimize the care of people with epilepsy and gestational capacity throughout pregnancy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sazgar M. Treatment of women with epilepsy. Continuum. 2019;2(2):408–30.
•• Tomson T, Battino D, Bromley R, et al. Executive summary: management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on women and pregnancy. Epilepsia. 2019;21:497–517. This task force summarizes current recommendations based on available data for managing epilepsy in pregnancy. This includes expert consensus when data is limited.
Tomson T, Battino D, Perucca E. Teratogenicity of antiepileptic drugs. Curr Opin Neurol. 2019;32(2):246–52. https://doi.org/10.1097/WCO.0000000000000659.
Hernández-Díaz S, Smith CR, Shen A, for the North American AED Pregnancy Registry, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692–9.
Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry. 2014;85:1029–34. https://doi.org/10.1136/jnnp-2013-306318.
Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530–8.
Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11. https://doi.org/10.1002/14651858.CD010224.pub2.
• Hernandez-Diaz S, Huybrechts KF, Desai RJ, et al. Topiramate use early in pregnancy and the risk of oral clefts: a pregnancy cohort study. Neurology. 2018;90:342–51. https://doi.org/10.1212/WNL.0000000000004857. This study notes the elevated risk of cleft lip/cleft palette that is associated with topiramate, and is dose-dependent.
Hernandez-Diaz S, Mittendorf R, Smith C, et al. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet Gynecol. 2014;123(1):21–8. https://doi.org/10.1097/AOG.0000000000000018.
Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Brivaracetam add-on for refractory focal epilepsy: a systemic review and meta-analysis. Neurology. 2016;86:1344–52.
Paolini SL, Pilato M, Rajasekaran V, Waters JFR, Bagic A, Urban A. Outcomes in three cases after brivaracetam treatment during pregnancy. Acta Neurol Scand. 2020;141:438–41.
Landmark CJ, Rektorli L, Burns ML, et al. Pharmacokinetic data on brivaracetam, lacosamide, and perampanel during pregnancy and lactation. Epileptic Disord. 2021;23(2):426–31.
Zonegran. European Medicines Agency; 2023. https://www.ema.europa.eu/documents/product-information/zonegran-epar-product-information_en.pdf.
• Meador KJ, Pennell PB, May RC, et al. Fetal loss and malformations in the MONEAD study of pregnant women with epilepsy. Neurology. 2020;94(14):E1502–11. Severe pregnancy outcomes including fetal loss and malformations are higher in pregnancy in patients with epilepsy compared to healthy controls.
Kondo T, Kaneko S, Amano Y, Egawa I. Preliminary report on teratogenic effects of zonisamide in the offspring of treated women with epilepsy. Epilepsia. 1996;37:1242–4.
• McCluskey G, Kinney MO, Russell A, et al. Zonisamide safety in pregnancy: data from the UK and Ireland epilepsy and pregnancy register. Seizure. 2021;91:311–5. Higher rates of MCMs in infants exposed to zonisamide were found in this observational study compared to what was previously reported, which highlights the need for further study about the teratogenicity of zonisamide.
Holmes L. Annual update for 2022. The North American Antiepileptic Drug Pregnancy Registry; 2022. https://www.aedpregnancyregistry.org/annual-update-for-2022.
Vazquez B, Tomson T, Dobrinsky C, et al. Perampanel and pregnancy. Epilepsia. 2021;62:698–708. https://doi.org/10.1111/epi.16821.
Costa R, Magalhaes LM, Graca J, et al. Eslicarbazepine acetate exposure in pregnant women with epilepsy. Seizure. 2018;58:72–4. https://doi.org/10.1016/j.seizure.2018.04.007.
Zutshi D, Millis SR, Basha MM, Daimee MA, Srinivas M. Lacosamide serum concentrations during pregnancy. Epilepsy Behav. 2021;123:108253. https://doi.org/10.1016/j.yebeh.2021.108253.
Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866–72. https://doi.org/10.1212/WNL.0000000000001772.
•• Vajda FJE, O’Brien TJ, Graham JE, Hitchcock AA, Lander CM, Eadie MJ. Antiepileptic drug polytherapy in pregnant women with epilepsy. Acta Neurol Scand. 2018;138:115–21. https://doi.org/10.1111/ane.12965. The Australian Pregnancy Registry found that topiramate and valproate are often associated with the increased risk of MCMs seen in polytherapy.
Bromley RL, Calderbank R, Cheyne CP, et al. Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87:1943–53. https://doi.org/10.1212/WNL.0000000000003157.
• Knight R, Wittkowski A, Bromley RL. Neurodevelopmental outcomes in children exposed to newer anti-seizure medications: a systematic review. Epilepsia. 2021;62(8):1765–79. This systematic review summarizes the literature of cognitive outcomes with newer ASMs. Lamotrigine and levetiracetam have reassuring data about cognitive outcomes thus far.
Cohen MJ, Meador KJ, May R, et al. Fetal antiepileptic drug exposure and learning and memory functioning at 6 years of age: The NEAD prospective observational study. Epilepsy Behav. 2019;92:154–64.
• Unnikrishnan G, Jacob NS, Salim S et al. Enduring language deficits in children of women with epilepsy and the potential role of intrauterine exposure to antiepileptic drugs. Epilepsia. 2020;61(11):2442–51. Lower language scores were seen in children born to pregnant patients, particularly when exposed to valproate or phenobarbitone in utero.
• Meador KJ, Cohen M J, Loring DW, et al. Two-year-old cognitive outcomes in children of pregnant women with epilepsy in the maternal outcomes and neurodevelopmental effects of antiepileptic drugs study. JAMA Neurol. 2021;78(8):927–36. Cognitive outcomes at age 2 years were not different between children born to patients with epilepsy and healthy controls.
•• Bjørk M-H, Zoega H, Leinonen MK, et al. Association of prenatal exposure to anti-seizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672–81. https://doi.org/10.1001/jamaneurol.2022.1269. This cohort study found that in-utero exposure to valproate and topiramate were associated with increased risk of neurodevelopmental disorders.
Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–52.
• Meador KJ, Pennell PB, May RC, et al. Effects of periconceptional folate on cognition in children of women with epilepsy: NEAD study. Neurology. 2020;94(7):e729–40. Perinatal folic acid supplementation is associated with higher full scale IQ scores in children of patients with epilepsy, highlighting the protective effects of folate.
Husebye ESN, Gilhus NE, Riedel B, et al. Verbal abilities in children of mothers with epilepsy. Neurology. 2018;91(9):e811–21.
Husebye ESS, Gilhus NE, Spigset O, et al. Language impairment in children aged 5 and 8 years after antiepileptic drug exposure in utero – the Norwegian Mother and Child Cohort Study. Eur J Neurol. 2020;27(4):667–75.
Dwyer ER, Filion KB, MacFarlane AJ, Platt RW, Mehrabadi A. Who should consume high-dose folic acid supplements before and during early pregnancy for prevention of neural tube defects? BMJ. 2022;377:3067728.
Valera-Gran D, Garcia de la Hera M, Navartete-Munoz EM, Fernandez-Somoano A, et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014;168(11):e142611.
• Compan Gabucio LM, Garcia de la Hera M, Torres Collado L, Fernandez-Somoano A, et al. The use of lower or higher than recommended doses of folic acid supplements during pregnancy is associated with child attentional dysfunction at 4–5 years of age in the INMA Project. Nutrients. 2021;13:327–43. Low dose (<400 ug/day) and high dose (>1000 ug/day) were associated with attention problems in children at age 4-5 years old, particularly male children.
•• Vegrim HM, Dreier JW, Alvestad S, et al. Cancer risk in children of mothers with epilepsy and high-dose folic acid use during pregnancy. JAMA Neurol. Published online September 26, 2022. https://doi.org/10.1001/jamaneurol.2022.2977. This review found increased risk of childhood cancers in children exposed to high dose folic acid (defined as >1 mg) compared to those on low dose folic acid born to patients with epilepsy. This raises concern that high dose folic acid may be detrimental.
Pennell PB and Hovinga CA. Chapter 13 Antiepileptic drug therapy in Pregnancy I: gestation-induced effects on AED pharmacokinetics. International Review of Neurobiology, Academic Press, Volume 83, 2008, 227–240.
•• Pennell PB, Karanam A, Meador K, et al. Anti-seizure medication concentrations during pregnancy: results from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. JAMA Neurol. 2022;79:370–9. This prospective cohort study found that dose-normalized concentrations of ASMs including lamotrigine, levetiracetam, lacosamide, oxcarbazepine, and zonisamide all significantly decreased during pregnancy.
La Neve A, Boero G, Francavilla T, et al. Prospective, case–control study on the effect of pregnancy on seizure frequency in women with epilepsy. Neurol Sci. 2015;36(1):79–83.
Schmidt J, Canger R, Avanzini G, et al. Change of seizure frequency in pregnant epileptic women. J Neurol. 1983;46:751–5.
The EURAP Study Group. Seizure control and treatment changes in pregnancy: Observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66(3):354–60.
Battino D, Tomson T, Bonizzoni E, et al. Seizure control and treatment changes in pregnancy: Observations from the EURAP epilepsy pregnancy registry. Epilepsia. 2013;54(9):1621–7.
•• Pennell PB, French JA, May RC, et al. Changes in seizure frequency and antiepileptic therapy during pregnancy. New Engl J Med. 2020;383(26):2547–56. There was not a significant difference in seizure frequency between pregnant patients and non-pregnant controls over in this prospective observational cohort study. However, doses of seizure medications were changed more often in pregnant patients.
Baishya J, Jose M, Reshma AS, Sarma PS, Thomas SV. Do women with epilepsy benefit from epilepsy specific pre-conception care? Epilepsy Res. 2020;160:1–4.
Ip S, Chung M, Raman G, Trikalinos TA, Lau J. A summary of the agency for healthcare research and quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4(SPECIAL ISSUE).
Al-Faraj AO, Pandey S, Herlihy MM, Pang TD. Factors affecting breastfeeding in women with epilepsy. Epilepsia. 2021;62(9):2171–9.
Shawahna R, Zaid L. Concentrations of anti-seizure medications in breast mild of lactating women: a systematic review with qualitative synthesis. Seizure. 2022;98:57–70.
•• Birnbaum AK, Meador KJ, Karanam A, et al. Antiepileptic drug exposure in infants of breastfeeding mothers with epilepsy. JAMA Neurol. 2020;77(4):441–50. This prospective cohort study evaluates the concentration of ASM in infant serum compared to mother’s serum during breastfeeding. In general, ASM concentrations were much lower in infants compared to mothers, lending support to breastfeeding for lactating patients with epilepsy.
Vajda FJE, Graham JE, Hitchcock AA, Lander CM, O’Brien TJ, Eadie MJ. Antiepileptic drugs and foetal malformation: analysis of 20 years of data in a pregnancy register. Seizure. 2019;65:6–11. https://doi.org/10.1016/j.seizure.2018.12.006. Elsevier online serial.
Thomas SV, Jeemon P, Pillai R, et al. Malformation risk of new anti-epileptic drugs in women with epilepsy; observational data from the Kerala registry of epilepsy and pregnancy (KREP). Seizure. 2021;93:127–32. https://doi.org/10.1016/j.seizure.2021.10.015. Elsevier online serial.
Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–9 Online serial.
Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–8.
Hunt S, Russell A, Smithson WH, et al. Topiramate in pregnancy: preliminary experience from the UK epilepsy and pregnancy register. Neurology. 2008;71:272–6.
Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–8 Online serial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Gerard reports grant support from being clinical trial PI: Eisai, Sunovion, Xenon, NINDS (U01-NS038455; 2U01-NS038455). She has received support for lectures from Neurology Week and Greenwich Pharmaceuticals. Dr. King reports grant support from Eisei Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
King, A., Gerard, E.E. Updates on Anti-seizure Medication Use in Pregnancy. Curr Obstet Gynecol Rep 12, 37–44 (2023). https://doi.org/10.1007/s13669-023-00359-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-023-00359-6