Abstract
Purpose of the Review
The purpose of this review is to provide a synopsis of all the mechanisms involved in the pathogenesis of adenomyosis. It will summarize recent advances in the field, discussing current controversies, and considering potential future directions.
Recent Findings
Adenomyosis pathogenesis is still a topic under investigation, however advancements in the understanding of disease development and mechanisms have been made. New data coming from new next generation sequencing-based studies and more-in-depth acquisitions on sex hormones imbalance, neuroangiogenesis, inflammation, fibrosis and cell proliferation have been obtained.
Summary
Adenomyosis is a uterine disorder that affects women of reproductive age, characterized by a benign invasion of the endometrium basalis (glands and stroma) within the myometrium.
So far, three theories for the pathophysiology of adenomyosis have been proposed:
-
1.
An invagination of the endometrial basalis into the myometrium by tissue injury and repair.
-
2.
The development from adult stem cells or displaced embryonic müllerian remnants.
-
3.
An “invasion from outside to inside”.
In order to invade and develop, endometrial cells require a series of pathogenetic mechanisms which drive to adenomyosis. Altered sex steroids hormones receptors may be the primary event which causes increased endometrial cell proliferations and differentiation from epithelial to mesenchymal cells. Once invaded the myometrium, an inflammatory reaction is displayed, probably driven by local immune changes. The processes of neuroangiogenesis and fibrosis are also involved in the adenomyosis development and may explain some of the associated clinical symptoms (dysmenorrhea, abnormal uterine bleeding, and infertility).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1860, Rokitansky was the first to recognize adenomyosis and to acknowledge the presence of endometrial glands and stromal cells within the myometrium (adenomyosis) and outside the uterine cavity (endometriosis). He denominated the two conditions respectively “endometriosis interna” and “endometriosis externa”. The first to use the term “adenomyosis” was Frankl [1], who used this compound word in 1925 (it is derived from the Greek phrases (αδέvας), gland, and mís (μυς), muscle) to describe a pathological disease of the muscular uterine tissue involving endometrial glands.
Adenomyosis is a benign estrogen-dependent uterine disorder that affects women of reproductive age. It is defined by the presence of endometrium basalis (glands and stroma) infiltration within the myometrium through an altered junctional zone (JZ), associated with myometrial hypertrophy/hyperplasia and fibrosis [1]. Several classifications have been developed and a number are still under development to describe adenomyosis by using histological or imaging criteria [2]. However, the most currently used classification describes three different phenotypes of adenomyosis: diffuse, when glands and stroma are dispersed throughout the myometrium; focal, when a nodular adenomyosis localization is identified, and adenomyoma (a cystic adenomyotic lesion) [3].
Aside from the diverse histological patterns, patients with adenomyosis suffer from dysmenorrhea, abnormal uterine bleeding, and infertility [4••]. In the past, adenomyosis was diagnosed predominantly in multiparous women, whereas nowadays it is identified also in young women of reproductive age due to the enhanced imaging techniques [4••, 5].
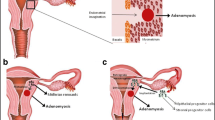
In order to explain the migration of endometrial cells and development of adenomyotic lesions within the myometrium, at least three theories are currently debated: a — “invasion from inside”, b — metaplasia of displaced embryonic pluripotent Mullerian remnants or adult stem cells, or c — “invasion from outside” [6, 7••].
In order to invade the myometrium, endometrial cells undergo a series of pathogenic processes which involve endocrine (gonadal sex steroid hormones), immune (inflammation), vascular (neoangiogenesis), and neuronal (neurogenesis) mechanisms [8, 9].
Theories on the Origin of Adenomyosis
Invagination of the Endometrial Basalis into the Myometrium
Contractions of the myometrium itself and the resultant trauma to the endometrial myometrial junction zone (JZ), a highly specialized hormone-responsive layer of the uterine architecture located in the inner third of the myometrium, may lead to the establishment of adenomyosis through invagination of the endometrium basalis into the myometrium [7••].
The TIAR theory (tissue injury and repair) was at the base of the Leyendecker’s “invagination theory” [8]. More specifically, in reaction to an injury/trauma, the TIAR system is activated, more estrogen is released locally due to local paracrine activity, boosting uterine contractility. A vicious loop is maintained, characterized by an increased release of estrogens, auto-traumatization and wound healing, which promotes inflammation and again a production of local estradiol [8, 9].
Besides, a new theory, named EMID (endometrial-myometrial interface disruption) has been proposed. This theory revises the tissue injury and healing theory, and claims that EMID caused by uterine surgeries might lead to a “iatrogenic” adenomyosis later in life [9, 10]. Indeed, a mechanical or thermal (as in electrocoagulation) elicitation of EMID is possible. Both modalities produce tissue damage, which stimulates substance P secretion that acts like an immune modulator, and activates the hypothalamic–pituitary–adrenal axis, resulting in an increase in catecholamine release, such as adrenaline/noradrenaline, which in turn may decrease cell-mediated immunity. Moreover, it was demonstrated that EMID, whether mechanically or thermally produced, may cause adenomyosis in mice, and the chance of inducing adenomyosis appears to be dependent on the severity of the EMID [10]. The EMID hypothesis includes epithelial mesenchymal transition, recruitment of bone-marrow-derived stem cells, and enhanced survival of endometrial cells dispersed and displaced due to iatrogenic procedures, in addition to hypoxia at the wounding site.
More crucially from a clinical point of view, considering the EMID hypothesis, specific perioperative therapies may minimize the development of adenomyosis [11].
Development from Adult Stem Cells
Adenomyotic lesions may also evolve indipendently and de novo from:
-
a)
Metaplasia of misplaced embryonic pluripotent Mullerian remnants in the myometrium. The Mullerian ducts are fundamental embryological structures that develop into the female uterine tract throughout fetal life. These ducts are constituted of surface epithelium and urogenital ridge mesenchyme, which can develop into endometrial glands and stroma.
-
b)
Multipotent adult stem cells differentiation (EnSCs) residing within the myometrium [9, 12]. These stem cells are hypothesized to reside within cell niches in the endometrium basalis to ensure cells regeneration and replacement in healthy endometrium. However, the presence of these cells may also promote unregulated proliferation that can extend beyond the endometrium.
Indeed, the capacity of a little portion of endometrium to reestablish the full functional layer is guaranteed by progenitor cells within the basal layer, where they restore glands, endometrial vasculature and stroma [12]. Menstrual blood-derived mesenchymal stem cells (MenSCs) may be displaced within the myometrium establishing de novo adenomyotic foci in a similar way how endometrial glands and stroma are formed [7••].
Invasion from Outside to Inside
Adult endometrial cells may be displaced into the myometrium as a result of the phenomenon of retrograde menstruation and the ability of ectopic endometrial cells to migrate and invade pelvic peritoneum. These cells seem to have the capability to invade the pelvic organs as well as the uterine walls and develop intra-myometrial endometrial implants, according to “from outside to inside invasion” theory [4••, 13]. The strong association between the posterior focal adenomyosis and deep infiltrating endometriosis nodules in the posterior compartment in endometriosis/adenomyosis patients supports the hypothesis [13] of a “from outside to inside invasion”, which refers to the displacement of endometrial cells into the myometrium from endometriosis lesions [13, 14].
Pathogenetic Mechanisms
Several mechanisms are involved in the pathogenesis of adenomyosis: impaired gonadal sex steroids hormones receptors function, altered cell proliferation and differentiation, inflammatory reaction, processes of neuroangiogenesis and fibrosis (Fig. 1).
The Role of Ovarian Sex Steroid Hormones
Estrogens and progesterone are the key regulators of healthy endometrium physiology to boost a regular menstrual cycle and create the perfect environment for embryo implantation.
Adenomyosis is promoted by an imbalance between estrogens and progesterone signaling in women during reproductive life [5, 7••, 15]. A high local production of estrogen, with normal peripheral levels of estradiol, has been shown in adenomyotic lesions, due to the high expression of aromatase [16, 17]. In fact, also high levels of estradiol in menstrual blood in comparison to normal serum levels have been shown in women with adenomyosis [7••, 17]. Furthermore, the gene polymorphism of aromatase cytochrome P450 has been found in the eutopic endometrium of patients with adenomyosis, and it is associated with a high local production of estrogen [18]. Polymorphisms of the estrogen receptor alpha (ERα) gene are also linked to an increased incidence of adenomyosis with a greater ER-beta expression in the myometrium of adenomyotic uteri, contributing to myometrial hyperplasia [15]. Furthermore, the modulation of 17b-hydroxysteroid dehydrogenase type 2 (an essential enzyme for the deactivation of estradiol to estrone) in the eutopic endometrium of women with adenomyosis differs compared to non-affected ones, as mRNA and 17bHSD2 activity are four- to six-fold higher in adenomyosis [17].
The increased estrogen activity stimulates the proliferative response, leading to changes in the expression of various other genes and may be related to the increased anti-apoptotic activity of the basalis, promoting the invagination process and the ‘spreading” of adenomyosis into the myometrium. Altered contractions stimulate the TIAR mechanism, resulting in enhanced estradiol production creating a loop that induces the invasion of the endometrial basalis into the myometrium and the formation of adenomyotic lesions [5, 7••].
The imbalance between estrogens and progesterone signaling is also caused by a decrease of progesterone activity, as suggested by the evidence that stromal cells of the functionalis and basalis endometrium of women with adenomyosis display a reduced immunoreactivity for isoform B of P receptor (PR-B), causing a loss of P effects [15]. As a result, estrogen-driven proliferative effects on the endometrium are not sufficiently counteracted by P during the secretory phase of the cycle, strengthening abnormal endometrial growth.
In addition, in ectopic endometrium, all three DNA Methyltransferases (DNMTs) are abnormally expressed, causing epigenetic changes. Disrupting either DNMT1 or DNMT3B alone seems to have a little effect on gene-specific methylation and related gene silencing in vitro. When both enzymes are disrupted, methyltransferase activity is almost inhibited, resulting in widespread chromosomal demethylation. In adenomyosis, immunoreactivity to DNMTs differs from that of normal endometrium, opening the scenario that adenomyosis is an epigenetic disorder [19]. These enzymes and several other mechanisms seem to be involved in the epigenetic regulation of ERs and PRs in patients with endometriosis, such as miRNA, transcriptional factors like GATA family, lncRNA [20]. However, additional studies are needed to state if these mechanisms are substantially involved also in adenomyosis.
New next generation sequencing (NGS)-based studies are showing that KRAS mutations, a cancer-associated gene, are more likely to be found in patients with adenomyosis and co-occurring endometriosis, causing inadequate PR expression [21]. KRAS activating mutations trigger particular pathways to enhance cell survival and proliferation, and are linked to progesterone resistance in adenomyosis [22••].
Cell Proliferation and Differentiation: Epithelial-to-Mesenchimal Transition (EMT)
The development of adenomyosis is related to the endometrial cells invasiveness of myometrium, and the epithelial-to-mesenchymal transition (EMT) is the one of the most accepted mechanism to support the changes undergoing ectopic endometrial cells. Epithelial cells leave their natural locus by detaching themselves from neighboring cells, change shape and migrate into the extracellular matrix of other tissues. This process is the mechanism by which cancer cells infiltrate adjacent tissues. The EMT and a dysregulated immunological response are involved in the development of adenomyosis [23]. An overexpression of the EMT markers (fibronectin, n-cadherin, vimentin), loss of E-cadherin, loss of apical–basal cell polarity, a decrease in tight junction proteins and cytokeratin lead to a mesenchymal cell phenotype with the ability of endometrial cells to migrate and invade.
The mesenchymal phenotype is necessary to the cells to leave the epithelium and migrate, giving them a cancer-like phenotype and the opportunity to induce the disease. The ETM mechanisms are activated by an increased ER expression, downregulation of PRs and by platelet activation, in conjunction with a chronic hyperperistaltic activity [5, 9, 22••]. The platelet activation leads to hypoxia and an increase in the biosynthesis of estrogens in patients with adenomyosis, making possible the phenotypic change of the cell [24, 25].
Increased expression of Talin 1 mRNA levels has been linked to adenomyosis [26, 27]. Talin 1 is involved in cancerogenesis and in the activation of the EMT mechanism. Talin1, via activation of the canonical wnt/-catenin pathway, plays a role in inducing both EMT and increased migration and invasiveness in adenomyotic cells [26].
A comparison of proliferative endometrial transcriptomes from women with and without adenomyosis identified 140 upregulated and 884 downregulated genes in samples from those affected, as well as microRNAs of unclear importance. In particular, many miRNAs (like miR-124-3p or miR-145-5p) may have a role in enhancing the migration and epithelial-stromal transformation of endometrial stromal cells extracted from eutopic endometrium [28, 29].
Inflammation and Immunological Changes
Nowadays, adenomyosis is considered as a chronic inflammatory disease characterized by abundant inflammatory mediators both into adenomyotic lesions and in the peritoneal fluid [6, 8, 9, 11].
The relevant contribution of COX-2 and prostaglandin (PG) pathway in the pathogenesis of adenomyosis has been widely supported. In mice with experimental adenomyosis, the degree of muscularis infiltration in the endometrium was reduced after the treatment of celecoxib (a selective inhibitor of COX-2), thus suggesting a major role of COX2 in the disease [30]. An increased expression of COX2 and PGs is observed in the presence of corticotropin releasing hormone (CRH) and urocortin (UCN), two potent inflammatory peptides, whose mRNA expression is increased in patients with adenomyosis [31]. In addition, in adenomyotic uteri an imbalance between pro-inflammatory and anti-inflammatory cytokines was shown, as well as other immunological markers, with an increase in the levels of a number of pro-inflammatory factors (IL1b, IL6, IFNa, IFNc, TNFa, and others) and anti-inflammatory signals (IL-10, TGF-β) [11, 32]. This mechanism may produce a disrupted symmetry between pro-inflammatory and anti-inflammatory signals, linked with platelet activation that could consequently favors endometrial cell migration into the myometrium and EMT activation [25].
Moreover, in some recent studies, cannabinoids (CB), molecules that play a role in inflammation and in immunomodulation, have been taken into consideration. Two CB receptors, CB1 and CB2, are abnormally expressed in the endometrium and myometrium of patients with adenomyosis, suggesting a possible role of these molecules [33, 34].
Angiogenesis and Neurogenesis
Angiogenesis is a mechanism that involves the formation of new capillaries from pre-existing blood vessels and occurs physiologically in the proliferative phase of the menstrual cycle [35]. It was initially identified in several tumors, in which cells mutate and begin to produce angiogenic factors, thus implementing the angiogenic switch. An abnormal and intensified vascularization has been observed also in adenomyosis and, in this regard, estrogens promote cell mobilization and microvascular integration [35]. An increased neoangiogenesis in adenomyosis is confirmed by increased microvessel density both in ectopic and eutopic endometrium [36].
Vascular endothelial growth factor (VEGF), a strong endothelial cell mitogen highly secreted by endometrial epithelial, stromal, and perivascular cells in adenomyosis, is involved in this mechanism [37, 38]. It is a critical factor to regenerate the endometrial layer after menstruation but it has been shown to be over-expressed in patients with adenomyosis [35, 37, 38]. Hypoxia plays a direct role in increasing VEGF levels, leading to an angiogenesis activation in adenomyotic lesions and to abnormal uterine bleeding as a symptom. In particular, VEGF expression seems to be caused by an overexpression of hypoxia inducible factor (HIF-1) action in response to hypoxic stimuli [39].
Two other growth factors are actively involved in neoangiogenesis, follistatin and activin A, members of TGF-β family. They act as proangiogenic factors in adenomyosis, promoting the creation of new capillaries and increasing the surface of pre-existing capillaries when compared to controls. In particular, activin A increases the production of VEGF by endometrial stromal cells, modifying the vascularization and leading to the creation of new capillaries [22••, 31]. The mRNA expression of follistatin and activin type II receptors is also increased in adenomyotic nodules [31].
Neurogenesis also appears to be dysregulated in patients with adenomyosis. It is the process by which a coordinated growth of nerves occurs, regulated by estrogen, by immune mediators and other factors [5, 7••]. In fact, adenomyotic tissues express high levels of neurogenic factors, such as nerve growth factors (NGF), which regulates the secretion of inflammatory factors, contributing to dysmenorrhea and dyspareunia [40]. NGF production may be induced by hyperestrogenism itself, and it may cause mast cell growth and degranulation, producing inflammatory mediators. This leads to the production of peripheral nociceptors, increasing the perception of pain [39]. Conversely, inflammatory mediators, IL-1, TNF-B, largely increase NGF levels, supporting a connection between inflammatory and neurogenic pathways [40].
Fibrosis
Fibrosis is another mechanism involved in the pathogenesis of adenomyosis [7••, 11, 41]. The stiffness of the lesion appears to be related with the amount of fibrosis and with the intensity of painful symptoms in patients with adenomyosis [42].
Several factors may induce fibrosis in adenomyotic lesions. TGF-β family signaling modulates smooth muscle metaplasia and fibrosis, by acting via a Smad2/3-dependent signaling pathway. This mechanism gives the cells the ability to breakdown the ECM, facilitating their invasiveness throughout the myometrium gaining migratory features, such as loss of cell-cell attachment [43]. Myostatin and activin A are two TGF-β family members that control myometrial cell growth and promote muscle development. Myostatin is abundantly expressed in adenomyotic tissues and may be implicated, for this reason, in the hyperplasia of myometrial cells surrounding the adenomyotic lesions. An increased expression of these molecules are also observed in eutopic endometrium of patients with adenomyosis and in adenomyotic tissues supporting their involvement in the disease [44, 45].
Moreover, an upregulation of nuclear factor 2 (Nrf2) may cause intramyometrial migration of endometrial implants via MMP-9, which is involved in extracellular matrix breakdown. Other MMPs, such as MMP2 and MMP3, are also increased in the eutopic endometrium of adenomyosis patients, driving myometrial invasion by myometrial bundle cohesion loss. Also, Lysil Oxidase (LOX), an amine oxidase involved in the synthesis of connective tissue matrices, is involved in the myometrial invasion of endometrial cells because its downregulation in adenomyotic lesions results in a lower rigid ECM [28].
From a clinical point of view, transvaginal elastosonography seems to be one of the options to assess the stiffness of adenomyosis lesions, hence their fibrotic nature [46]. Higher lesional stiffness appears to be associated with reduced PR expression, suggesting that those patients may be potentially less likely to respond to progestin therapy [46].
Conclusions
The understanding of the pathogenetic mechanisms of adenomyosis may open new perspectives in developing new tools for the diagnosis and treatment, toward a more and more personalized medicine. Dysmenorrhea, abnormal uterine bleeding and subfertility are linked to different adenomyosis phenotypes and diverse underlying pathogenetic mechanisms [5, 7••, 47].
Dysmenorrhea seems to be linked to the amount of glandular tissue within the myometrium and the number of lesions foci. The degree of dysmenorrhea seems to be also linked to the expression of DNA Methyltransferases 3B opening the hypothesis that adenomyosis is an epigenetic disorder. [48]. Furthermore, women with adenomyosis-related menstrual pain have highly expressed markers of neurogenesis [49], suggesting that the increased extent of the local innervation triggers the pain symptoms [49].
AUB heavy menstrual bleeding is explained by an increased angiogenesis, whereas infertility seems to be related to a focal adenomyosis phenotype, along with endometrial abnormalities and altered decidualization process [50, 51]. Further studies are needed to have a better comprehension of all the mechanism involved in this disease, leading to a more precise diagnosis and treatment.
Data Availability
To review all information on pathogenic pathways of adenomyosis development and clinical presentation, a PubMed search of the literature from 1950 to January 2022 was conducted. All relevant publications were evaluated, along with their reference lists, to see if there were any further research that might be included.
Change history
22 July 2022
Open Access funding information has been added in the Funding Note.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Benagiano G, Brosens I. History of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):449–63.
Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111(4):629–40. Available from: https://doi.org/10.1016/j.fertnstert.2019.02.008.
Robbins. Student Consult [Internet]. 2015;1–1023. Available from: https://www.papers3.com/publication/uuid/5EA1AA7A-8B6E-424A-B367-0DC1E86B0C47.
•• Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26(3):392–411. An update review on the different approaches to adenomyosis diagnosis
Vannuccini S, Tosti C, Carmona F, Huang SJ, Chapron C, Guo SW, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35(5):592–601. Available from: https://doi.org/10.1016/j.rbmo.2017.06.016.
Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it’s time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2020.
•• Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: Mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2–3):129–43. In this review mechanism and pathogenesis of adenomyosis are elucidated highlighting the importance of integrating existing knowledge with novel models, approaches, technologies, and big data.
Leyendecker G, Wildt L. A new concept of endometriosis and adenomyosis: tissue injury and repair (TIAR). Horm Mol Biol Clin Investig. 2011;5(2):125–42. Available from: https://www.degruyter.com/document/doi/10.1515/HMBCI.2011.002/html.
García-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril [Internet]. 2018;109(3):371–9. Available from: https://doi.org/10.1016/j.fertnstert.2017.12.030.
Hao M, Liu X, Guo SW. Adenomyosis in mice resulting from mechanically or thermally induced endometrial-myometrial interface disruption and its possible prevention. Reprod Biomed Online [Internet]. 2020;41(5):925–42. Available from: https://pubmed.ncbi.nlm.nih.gov/32921577/.
Guo SW. The pathogenesis of adenomyosis vis-à-vis endometriosis. J Clin Med. 2020;9(2).
Gurung S, Deane JA, Masuda H, Maruyama T, Gargett CE. Stem cells in endometrial physiology. Semin Reprod Med. 2015;33(5):326–32.
Chapron C, Tosti C, Marcellin L, Bourdon M, Lafay-Pillet MC, Millischer AE, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32(7):1393–401.
Bourdon M, Santulli P, Jeljeli M, Vannuccini S, Marcellin L, Doridot L, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. 2021;27(1):108–29.
Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril [Internet]. 2011;95(7):2228–2235.e1. Available from: https://doi.org/10.1016/j.fertnstert.2011.02.051.
J K, T N, T A, K M, K T, T Y, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod [Internet]. 1997;57(3):514–9. Available from: https://pubmed.ncbi.nlm.nih.gov/9282984/.
Kitawaki J. Adenomyosis: the pathophysiology of an oestrogen-dependent disease. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):493–502.
Tong X, Li Z, Wu Y, Fu X, Zhang Y, Fan H. COMT 158G/A and CYP1B1 432C/G polymorphisms increase the risk of endometriosis and adenomyosis: a meta-analysis. Eur J Obstet Gynecol Reprod Biol [Internet]. 2014;179:17–21. Available from: https://doi.org/10.1016/j.ejogrb.2014.04.039.
Guo XLS. Aberrant immunoreactivity of Deoxyribonucleic Acid Methyltransferases in Adenomyosis. 2012;100–8.
Chen H, Malentacchi F, Fambrini M, Harrath AH, Huang H, Petraglia F. Epigenetics of estrogen and progesterone receptors in endometriosis. Reprod Sci 2020 2711 [Internet]. 2020 Jul 22 [cited 2022 Jan 8];27(11):1967–74. Available from: https://link.springer.com/article/https://doi.org/10.1007/s43032-020-00226-2.
Inoue S, Hirota Y, Ueno T, Fukui Y, Yoshida E, Hayashi T, et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat Commun [Internet]. 2019; Available from: https://doi.org/10.1038/s41467-019-13708-y.
•• Bulun SE, Yildiz S, Adli M, Wei J-J. Adenomyosis pathogenesis: insights from next-generation sequencing. Hum Reprod Update [Internet]. 2021 Jun 15 [cited 2021 Dec 12];27(6). Available from: https://pubmed.ncbi.nlm.nih.gov/34131719/. Findings from this study suggest that Kras, a cancer-associated gene, plays a role in the pathogenesis of adenomyosis. It also highlights the importance of the use of new techniques like NGS studies to provide the best scientific evidence about the cellular origins of adenomyosis and the contributions of new signaling pathways to its pathogenesis.
Bourdon M, Santulli P, Doridot L, Jeljeli M, Chêne C, Chouzenoux S, et al. Immune cells and Notch1 signaling appear to drive the epithelial to mesenchymal transition in the development of adenomyosis in mice. Mol Hum Reprod [Internet]. 2021 Oct 1 [cited 2021 Dec 12];27(10). Available from: https://pubmed.ncbi.nlm.nih.gov/34463756/.
Qi Q, Liu X, Zhang Q, Guo SW. Platelets induce increased estrogen production through NF-κB and TGF-β1 signaling pathways in endometriotic stromal cells. Sci Rep. 2020;10(1).
Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum Reprod [Internet]. 2016;31(4):734–49. Available from: https://pubmed.ncbi.nlm.nih.gov/26908845/.
Wang Y, Duan H, Wang S, Quan Y, Huang J, Guo Z. Upregulated Talin1 synergistically boosts β - estradiol-induced proliferation and pro- angiogenesis of eutopic and ectopic endometrial stromal cells in adenomyosis. 2021;7:1–15.
Wang Y yi, Duan H, Wang S, Quan Y jun, Huang J hua, Guo Z chen. Talin1 Induces epithelial-mesenchymal transition to facilitate endometrial cell migration and invasion in adenomyosis under the regulation of microRNA-145–5p. Reprod Sci [Internet]. 2021;28(5):1523–39. Available from: https://pubmed.ncbi.nlm.nih.gov/33537874/.
Herndon CN, Aghajanova L, Balayan S, Erikson D, Barragan F, Goldfien G, et al. Global Transcriptome Abnormalities of the Eutopic Endometrium From Women With Adenomyosis. 2016;23(10):1289–303.
Huang N, Xu L, Qiu Y, Zhan J, Chen X. Tissue and Cell Down-regulated miR-124–3p enhanced the migration and epithelial-stromal transformation of endometrial stromal cells extracted from eutopic endometrium in subjects with adenomyosis by up-regulating Neuropilin 1. Tissue Cell [Internet]. 2021;69(126):101474. Available from: https://doi.org/10.1016/j.tice.2020.101474.
Liang S, Shi L, Duan J, Liu H, Wang T, Li C. Celecoxib reduces inflammation and angiogenesis in mice with adenomyosis. 2021;13(4):2858–66.
Carrarelli P, Luddi A, Funghi L, Arcuri F, Batteux F, Dela Cruz C, et al. Urocortin and corticotrophin-releasing hormone receptor type 2 mRNA are highly expressed in deep infiltrating endometriotic lesions. Reprod Biomed Online [Internet]. 2016;33(4):476–83. Available from: http://www.rbmojournal.com/article/S1472648316304400/fulltext.
Bourdon M, Santulli P, Jeljeli M, Vannuccini S, Marcellin L, Doridot L, et al. Immunological changes associated with adenomyosis : a systematic review. 2020;1–22.
Shen X, Duan H, Wang S, Hong W, Wang YY, Lin SL. Expression of cannabinoid receptors in myometrium and its correlation with dysmenorrhea in adenomyosis. Reprod Sci [Internet]. 2019;26(12):1618–25. Available from: https://doi.org/10.1177/1933719119833483.
Shen X, Duan H, Wang S, Gan L, Xu Q, Li J-J, et al. Decreased Expression of Cannabinoid Receptors in the Eutopic and Ectopic Endometrium of Patients with Adenomyosis. 2019. Available from:https://doi.org/10.1155/2019/5468954.
Harmsen MJ, Wong CFC, Mijatovic V, Griffioen AW, Groenman F, Hehenkamp WJK, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update. 2019;25(5):647–71.
Schindl M, Birner P, Obermair A, Kiesel L, Wenzl R. Increased microvessel density in adenomyosis uteri. Fertil Steril [Internet]. 2001;75(1):131–5. Available from: https://pubmed.ncbi.nlm.nih.gov/11163827/.
Filippi I, Carrarelli P, Luisi S, Batteux F, Chapron C, Naldini A, et al. Different expression of hypoxic and angiogenic factors in human endometriotic lesions. Reprod Sci [Internet]. 2016;23(4):492–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26408396/.
Yalaza C, Canacankatan N, Gürses İ, Aytan H, Taşdelen B. Altered VEGF, Bcl-2 and IDH1 expression in patients with adenomyosis. Arch Gynecol Obstet [Internet]. 2020;302(5):1221–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32785780/.
Goteri G, Lucarini G, Montik N, Zizzi A, Stramazzotti D, Fabris G, et al. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol. 2009;28(2):157–63.
Luddi A, Marrocco C, Governini L, Semplici B, Pavone V, Capaldo A, et al. Increased expression of neurogenic factors in uterine fibroids. Hum Reprod. 2019;34(11):2153–62.
Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Research [Internet]. 2019;8. Available from: /pmc/articles/PMC6419978/
Stoelinga B, Hehenkamp WJK, Nieuwenhuis LL, Conijn MMA, van Waesberghe JHHTM, Brölmann HAM, et al. Accuracy and reproducibility of sonoelastography for the assessment of fibroids and adenomyosis, with magnetic resonance imaging as reference standard. Ultrasound Med Biol [Internet]. 2018;44(8):1654–63. Available from: https://doi.org/10.1016/j.ultrasmedbio.2018.03.027.
Cheong ML, Lai TH, Wu W Bin. Connective tissue growth factor mediates transforming growth factor β-induced collagen expression in human endometrial stromal cells. PLoS One [Internet]. 2019;14(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30695033/.
Carrarelli P, Yen CF, Arcuri F, Funghi L, Tosti C, Wang TH, et al. Myostatin, follistatin and activin type II receptors are highly expressed in adenomyosis. Fertil Steril [Internet]. 2015;104(3):744–752.e1. Available from: https://doi.org/10.1016/j.fertnstert.2015.05.032.
Carrarelli P, Funghi L, Ciarmela P, Centini G, Reis FM, Dela Cruz C, et al. Deep infiltrating endometriosis and endometrial adenocarcinoma express high levels of myostatin and its receptors messenger RNAs. Reprod Sci. 2017;24(12):1577–82.
Liu X, Ding D, Ren Y, Guo SW. Transvaginal elastosonography as an imaging technique for diagnosing adenomyosis. Reprod Sci. 2018;25(4):498–514.
Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update [Internet]. 2012;18(4):374–92. Available from: https://pubmed.ncbi.nlm.nih.gov/22442261/.
Liu X, Guo S-W. Aberrant immunoreactivity of deoxyribonucleic acid methyltransferases in adenomyosis. Gynecol Obstet Invest. 2012;74(2):100–8.
Orazov MR, Radzinsky VE, Nosenko EN, Khamoshina MB, Dukhin AO, Lebedeva MG. Immune-inflammatory predictors of the pelvic pain syndrome associated with adenomyosis. Gynecol Endocrinol. 2017;33:44–6.
Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95(5):688–91.
Nishida M. Relationship between the onset of dysmenorrhea and histologic findings in adenomyosis. Am J Obstet Gynecol [Internet]. 1991;165(1):229–31. Available from: https://doi.org/10.1016/0002-9378(91)90257-R.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Uterine Fibroids and Endometrial Lesions
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rossi, M., Vannuccini, S., Capezzuoli, T. et al. Mechanisms and Pathogenesis of Adenomyosis. Curr Obstet Gynecol Rep 11, 95–102 (2022). https://doi.org/10.1007/s13669-022-00326-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-022-00326-7