Abstract
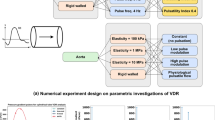
To investigate the effects of pulsatile and continuous pediatric ventricular assist (PVAD) flow and pediatric blood viscoelasticity on hemodynamics in a pediatric aortic graft model. Hemodynamic parameters of pulsatility, along with velocity and wall shear stress (WSS), are analyzed and compared between Newtonian and viscoelastic blood models at a range of physiological pediatric hematocrits using computational fluid dynamics. Both pulsatile and continuous PVAD flow lead to a decrease in pulsatility (surplus hemodynamic energy, ergs/cm3) compared to healthy aortic flow but with continuous PVAD pulsatility up to 2.4 times lower than pulsatile PVAD pulsatility at each aortic outlet. Significant differences are also seen between the two flow modes in velocity and WSS. The higher velocity jet during systole with pulsatile flow leads to higher WSSs at the anastomotic toe and at the aortic branch bifurcations. The lower velocity but continuous flow jet leads to a much different flow field and higher WSSs into diastole. Under a range of physiological pediatric hematocrit (20–60%), both velocity and WSS can vary significantly with the higher hematocrit blood model generally leading to higher peak WSSs but also lower WSSs in regions of flow separation. The large decrease in pulsatility seen from continuous PVAD flow could lead to complications in pediatric vascular development while the high WSSs during peak systole from pulsatile PVAD flow could lead to blood damage. Both flow modes lead to similar regions prone to intimal hyperplasia resulting from low time-averaged WSS and high oscillatory shear index.












Similar content being viewed by others
References
Almond, C., D. Morales, E. Blackstone, M. Turrentine, M. Imamura, M. Massicotte, L. Jordan, et al. Berlin heart excor pediatric ventricular assist device for bridge to heart transplantation in us children. Circulation 127(16):1702–1711, 2013.
Baldwin, J., H. Borovetz, and B. Duncan. The national heart, lung, and blood institute pediatric circulatory support program. Circulation 113(1):147–155, 2006.
Bingyang, J., and A. Undar. An evaluation of the benefits of pulsatile versus nonpulsatile perfusion during cardiopulmonary bypass procedures in pediatric and adult cardiac patients. ASAIO J. 52(4):357–361, 2006.
Bodnár, T., A. Sequeira, and M. Prosi. On the shear-thinning and viscoelastic effects of blood flow under various flow rates. Appl. Math. Model. 217(11):5055–5067, 2011.
Chen, J., X. Lu, and W. Wang. Non-Newtonian effects of blood flow on hemodynamics in distal vascular graft anastomoses. J. Biomech. 39(11):1983–1995, 2006.
Chiang, B., C. Ye, X. Gou, Y. Gu, Y. Zhou, J. Hong, and Y. Wang. Effects of pulsatile reperfusion on globally ischemic myocardium. ASAIO J. 39:438–443, 1993.
Connell, J., T. Khalapyan, J. Myers, G. Rosenberg, and W. Weiss. Anastomotic Fit Assessment for the Penn State Pediatric Ventricular Assist Device. ASAIO J. 53:687–691, 2007.
Favero, J., A. Secchi, N. Cardozo, and H. Jasak. Viscoelastic flow analysis using the software OpenFOAM and differential constitutive equations. J Non-Newton Fluid. 165:23–24, 2010.
Fogel, M., P. Weinberg, J. Rychik, A. Hubbard, M. Jacobs, T. Spray, and J. Haselgrove. Caval contribution to flow in the branch pulmonary arteries of fontan patients with a novel application of magnetic resonance presaturation pulse. Circulation 99(9):1215–1221, 1999.
Fukae, K., R. Tominaga, S. Tokunaga, Y. Kawachi, T. Imaizumi, and H. Yasui. The effects of pulsatile and nonpulsatile systemic perfusion on renal sympathetic nerve activity in anesthetized dogs. J. Thorac. Cardiovasc. Surg. 111:478–484, 1996.
Gaer, J., A. Shaw, R. Wild, R. Swift, C. Munsch, P. Smith, and K. Taylor. Effect of cardiopulmonary bypass on gastrointestinal perfusion and function. Ann. Thorac. Surg. 57:371–375, 1994.
Glor, F., B. Ariff, A. Hughes, L. Crowe, P. Verdonck, D. Barratt, S. McG Thom, D. Firmin, and X. Xu. Image-based carotid flow reconstruction: a comparison between MRI and ultrasound. Physiol. Meas. 25(6):1495, 2004.
Good, B. C., S. Deutsch, K. B. Manning. Hemodynamics in a pediatric ascending aorta using a viscoelastic pediatric blood model. Ann. Biomed. Eng. 2015. doi:10.1007/s10439-015-1370-z.
Huo, Y., X. Guo, and G. Kassab. The flow field along the entire length of mouse aorta and primary branches. Ann. Biomed. Eng. 36(5):685–699, 2008.
Issa, R. Solution of the implicitly discretised fluid flow equations by operator-splitting. J. Comput. Phys. 62:40–65, 1986.
Johnston, B., P. Johnston, S. Corney, and D. Kilpatrick. Non-Newtonian blood flow in human right coronary arteries: steady state simulations. J. Biomech. 37(5):709–720, 2004.
Johnston, B., P. Johnston, S. Corney, and D. Kilpatrick. Non-Newtonian blood flow in human right coronary arteries: transient simulations. J. Biomech. 39(6):1116–1128, 2006.
Jopling, J., E. Henry, S. Wiedmeier, and R. Christensen. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics 123(2):333–337, 2009.
Kent, A., Z. Kecskes, B. Shadbolt, and M. Falk. Blood pressure in the first year of life in healthy infants born at term. Pediatr. Nephrol. 22(10):1743–1749, 2007.
Kohler, T., T. Kirkman, L. Kraiss, B. Zierler, and A. Clowes. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ. Res. 69:1557–1565, 1991.
Ku, D., D. Giddens, C. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscl. Throm. Vas. 5(3):293–302, 1985.
Leuprecht, A., and K. Perktold. Computer simulation of non-Newtonian effects on blood flow in large arteries. Comput. Methods Biomech. 4(2):149–163, 2001.
Leverett, L., J. Hellums, C. Alfrey, and E. Lynch. Red blood cell damage by shear stress. Biophys. J. 12(3):257, 1972.
Linderkamp, O., H. Versmold, K. Riegel, and K. Betke. Contributions of red cells and plasma to blood viscosity in preterm and full-term infants and adults. Pediatrics 74(1):45–51, 1984.
LoGerfo, F., W. Quist, M. Nowak, H. Crawshaw, and C. Haudenschild. Downstream anastomotic hyperplasia. A mechanism of failure in Dacron arterial grafts. Ann. Surg. 197(4):479, 1983.
Long, J., A. Undar, K. Manning, and S. Deutsch. Viscoelasticity of pediatric blood and its implications for the testing of a pulsatile pediatric blood pump. ASAIO J. 51(5):562–566, 2005.
Loth, F., P. Fischer, and H. Bassiouny. Blood flow in end-to-side anastomoses. Annu. Rev. Fluid Mech. 40:367–393, 2008.
Loth, F., S. Jones, C. Zarins, D. Giddens, R. Nassar, S. Glagov, and H. Bassiouny. Relative contribution of wall shear stress and injury in experimental intimal thickening at PTFE end-to-side arterial anastomoses. J. Biomech. Eng. 124(1):44–51, 2002.
Machii, M., and A. Becker. Morphologic features of the normal aortic arch in neonates, infants, and children pertinent to growth. Ann. Thorac. Surg. 64(2):511–515, 1997.
Mah, D., T. Singh, R. Thiagarajan, K. Gauvreau, G. Piercey, E. Blume, F. Fynn-Thompson, and C. Almond. Incidence and risk factors for mortality in infants awaiting heart transplantation in the USA. J. Heart Lung Transpl. 28(12):1292–1298, 2009.
Malek, M., S. Alper, and S. Izumo. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA-J Am Med Assoc. 282(21):2035–2042, 1999.
Matoth, Y., R. Zaizov, and I. Varsano. Postnatal changes in some red cell parameters. Acta Paediatr. 60:317–323, 1971.
Matsumoto, T., C. Wolferth, and M. Perlman. Effects of pulsatile and non-pulsatile perfusion upon cerebral and conjunctival microcirculation in dogs. Am. Surg. 37:61–64, 1971.
May-Newman, K., B. Hillen, and W. Dembitsky. Effect of left ventricular assist device outflow conduit anastomosis location on flow patterns in the native aorta. ASAIO J. 52(2):132–139, 2006.
Mori, A., R. Tabata, Y. Nakamura, K. Watanabe, M. Onoe, and Y. Okada. Effects of pulsatile cardiopulmonary bypass on carbohydrate and lipid metabolism. J. Cardiovasc. Surg. 28:621–626, 1987.
Nagaoka, H., R. Innami, M. Watanabe, M. Satoh, F. Murayama, and N. Funakoshi. Preservation of pancreatic beta cell function with pulsatile cardiopulmonary bypass. Ann. Thorac. Surg. 48:798–802, 1989.
O’Callaghan, S., M. Walsh, and T. McGloughlin. Numerical modelling of Newtonian and non-Newtonian representation of blood in a distal end-to-side vascular bypass graft anastomosis. Med. Eng. Phys. 28(1):70–74, 2006.
Ojha, M. Spatial and temporal variations of wall shear stress within an end-to-side arterial anastomosis model. J. Biomech. 26(12):1377–1388, 1993.
Pantalos, G., G. Giridharan, J. Colyer, M. Mitchel, J. Speakman, C. Lucci, G. Johnson, M. Gartner, and S. Koenig. Effect of continuous and pulsatile left ventricular assist on pulsatility in a pediatric animal model of left ventricular dysfunction: pilot observations. ASAIO J. 53(3):385–391, 2007.
Pekkan, K., O. Dur, K. Sundareswaran, K. Kanter, M. Fogel, A. Yoganathan, and A. Undar. Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass. J. Biomech. Eng. 130(6):061012, 2008.
Pekkan, K., D. Frakes, D. De Zelicourt, C. Lucas, J. Parks, and A. Yoganathan. Coupling pediatric ventricle assist devices to the fontan circulation: simulations with a lumped-parameter model. ASAIO J. 51(5):618–628, 2005.
Rajagopal, K., and A. Srinivasa. A thermodynamic framework for rate-type fluid models. J Non-Newton Fluid. 88(3):207–227, 2000.
Shahcheraghi, N., H. Dwyer, A. Cheer, I. Barakat, and T. Rutaganira. Unsteady and three-dimensional simulation of blood flow in the human aortic arch. J. Biomech. Eng. 124(4):378–387, 2002.
Shepard, R., D. Simpson, and J. Sharp. Energy Equivalent Pressure. Arch. Surg. 93:730–740, 1966.
Silverman, N., S. Levitsky, J. Kohler, M. Trenkner, and H. Feinberg. Prevention of reperfusion injury following cardioplegic arrest by pulsatile flow. Ann. Thorac. Surg. 35(5):493–499, 1983.
Taylor, K., W. Bain, K. Maxted, M. Hutton, W. McNab, and P. Caves. Comparative studies of pulsatile and nonpulsatile flow during cardiopulmonary bypass. I. Pulsatile system employed and its hematologic effects. J. Thorac. Cardiovasc. Surg. 75:569–573, 1978.
Travis, A., G. Giridharan, G. Pantalos, R. Dowling, S. Prabhu, M. Slaughter, M. Sobieski, A. Ündar, D. Farrar, and S. Koenig. Vascular pulsatility in patients with a pulsatile-or continuous-flow ventricular assist device. J. Thorac. Cardiovasc. Surg. 133(2):517–524, 2007.
Trinkle, J., N. Helton, R. Wood, and L. Bryant. Metabolic comparison of a new pulsatile pump and a roller pump for cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 58:562, 1969.
Ündar, A., and C. Fraser, Jr. Defining pulsatile perfusion: quantification in terms of energy equivalent pressure. Artif. Organs 23:712–716, 1999.
Ündar, A., N. Henderson, G. Thurston, T. Masai, E. Beyer, and C. Fraser, Jr. The effects of pulsatile versus nonpulsatile perfusion on blood viscoelasticity before and after deep hypothermic circulatory arrest in a neonatal piglet model. Artif. Organs 23:717–721, 1999.
Ündar, A., T. Masai, E. Beyer, J. Goddard-Finegold, M. McGarry, and C. Fraser, Jr. Pediatric physiologic pulsatile pump enhances cerebral and renal blood flow during and after cardiopulmonary bypass. Artif. Organs 26:919–923, 2002.
Van der Linde, D., E. Konings, M. Slager, M. Witsenburg, W. Helbing, J. Takkenberg, and J. Roos-Hesselink. Birth prevelance of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 58(21):2241–2247, 2011.
Weiss, W., B. Lukic, and A. Ündar. Energy equivalent pressure and total hemodynamic energy associated with the pressure-flow waveforms of a pediatric pulsatile ventricular assist device. ASAIO J. 51(5):614–617, 2005.
Wesolowski, S., L. Sauvage, and R. Pinc. Extracorporeal circulation: the role of the pulse in maintenance of the systemic circulation during heart-lung by-pass. Surgery. 37(4):663–682, 1955.
Yang, N., S. Deutsch, E. Paterson, and K. Manning. Numerical study of blood flow at the end-to-side anastomosis of a left ventricular assist device for adult patients. J. Biomech. Eng. 131(11):111005, 2009.
Yang, N., S. Deutsch, E. Paterson, and K. Manning. Hemodynamics of an end-to-side anastomotic graft for a pulsatile pediatric ventricular assist device. J. Biomech. Eng. 132(3):031009, 2010.
Yang, N., S. Deutsch, E. Paterson, and K. Manning. Comparative study of continuous and pulsatile left ventricular assist devices on hemodynamics of a pediatric end-to-side anastomotic graft. Cardiovasc. Eng. Technol. 1(1):88–103, 2010.
Acknowledgments
We would like to acknowledge the National Institutes of Health for their support of this project through NIH NHLBI HL108123. Bryan C Good, Steven Deutsch, and Keefe B. Manning declare that they have no conflict of interest. No human or animal studies were carried out by the authors for this article. We also thank Ajit Yoganathan, PhD and Christopher M. Haggerty, PhD from the Department of Biomedical Engineering at the Georgia Institute of Technology for providing the pediatric aortic model.
Disclosures
The authors have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Good, B.C., Deutsch, S. & Manning, K.B. Continuous and Pulsatile Pediatric Ventricular Assist Device Hemodynamics with a Viscoelastic Blood Model. Cardiovasc Eng Tech 7, 23–43 (2016). https://doi.org/10.1007/s13239-015-0252-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-015-0252-8