Abstract
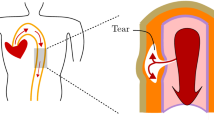
Aortic dissection and rupture may involve circumferential shear stress in the circumferential–longitudinal plane. Inflation of bovine descending aortic ring specimens provides evidence of such shear from the non-uniform circumferential distortion of radial lines drawn on the circumferential–radial ring face. Delamination without tensile peeling induces cracks that propagate nearly circumferentially in the circumferential–longitudinal plane from the root of a radial cut representing rupture initiation in a ring. Translational shear deformation tests of small rectangular aortic wall blocks in the circumferential and longitudinal direction measure the consequences of such shear on substructures in the aortic wall, in particular the collagen fibers. The two directions of shear deformation produce no statistical difference in the shear stress response of the wall. Possibly, the interfiber connections between collagen fibers are put into tension by either translational shear deformation so that the stress measured reflects the tensile response of these connections. Wall rupture may involve failure of these connections; such failure is supported by the voids parallel to the collagen fibers observed in a histological study after translational shear. Further, interstitial fluid is redistributed by shear as evidenced by the measured weight loss of a set of specimens during the translational shear of blocks. Because the mass changes, mathematical modeling of aortic tissue in vitro as incompressible is an approximation. These observations suggest that no simple modification of classical rupture theories, whether based on energy functions, stress or strain, suffices to predict the rupture of hydrated soft biological tissue that has complex substructures.












Similar content being viewed by others
References
Baek, S., R. L. Gleason, K. R. Rajagopal, and J. D. Humphrey. Theory of small on large: potential utility in computations of fluid–solid interactions in arteries. Comput. Methods Appl. Mech. Eng. 196:3070–3078, 2007.
Branchetti, E., P. Poggio et al. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc. Res. 100(2):316–324, 2013. doi:10.1093/cvr/cvt205.
Braverman, A. C. Acute aortic dissection: clinician update. Circulation 122:184–188, 2010. doi:10.1161/CIRCULATIONAHA.110.958975
Criado, F. J. Aortic dissection: a 250-year perspective. Tex. Heart Inst. J. 38(6):694–700, 2011.
Clark, J. M. and S. Glagov. Transmural organization of the arterial media. Arteriosclerosis 5:19–34, 1985.
Cohuet, G., P. Challande, M. Osborne-Pellegrin, S. M. Arribas, A. Dominiczak, H. Louis, S. Laurent, and P. Lacolley. Mechanical strength of the isolated carotid artery in SHR. Hypertension 38:1167–1171, 2001.
Davis, E. C. Smooth muscle cell to elastic lamella connections in developing mouse aorta. Role in aortic media organization. Lab. Invest. 68:89–99, 1993.
Dingemans, K. P., P. Teeling, J. H. Lagendijk, and A. E. Becker. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat. Rec. 258:1–14, 2000.
Groenink, M., S. E. Langerak, E. Wanbavel, E. van der Wall, B. Mulder, A. van der Wall, and J. Spaan. The influence of aging and aortic stiffness on permanent dilation and breaking stress of the thoracic descending aorta. Cardiovasc. Res. 43:471–480, 1999.
Haslach, H. W. Jr.. Time-dependent mechanisms in fracture of paper. Mech. Time Depend. Mater. 13:11–35, 2009. doi:10.1007/s11043-008-9074-5
Haslach, H. W. Jr., P. Riley and A. Molotsky. The influence of medial substructures on rupture in bovine aortas. Cardiovasc. Eng. Technol. 2(4):372–387, 2011. doi:10.1007/s13239-011-0056-4.
Holzapfel, G. A., T. C. Gasser, and R. W. Ogden. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 61:1–48, 2000.
Huang, Y., D. Rumschitzki, S. Chien, and S. Weinbaum. A fiber matrix model for the filtration through fenestral pores in a compressible arterial intima. Am. J. Physiol. 272(4):H2023–H2039, 1997.
Kamalakannan, D., H. S. Rosman, and K. A. Eagle. Acute aortic dissection. Crit. Care Clin. 23(40):779–800, 2007.
LeMaire, S. A. and L. Russell. Epidemiology of thoracic aortic dissection. Nat. Rev. Cardiol. 8:103–113, 2011. doi:10.1038/nrcardio.2010.187
Lillie, M. A. and J. M. Gosline. The effects of hydration on the dynamic mechanical properties of elastin. Biopolymers 29:1147–1160, 1990.
Mitchell, P. and J. Jakubowski. Failure testing cerebral arteries: are branch points weaker than unbranched vessels? Br. J. Neurosurg. 16:578–582, 2002.
Mohan, D. and J. W. Melvin. Failure properties of passive human aortic tissue. I—uniaxial tension tests. J. Biomech. 15:887–902, 1982.
NIH. What causes an aneurysm? http://www.nhlbi.nih.gov/health/health-topics/topics/arm/causes, 2015.
Pal, S., A.Tsamis, S. Pasta, A. D’Amore, T. G. Gleason, D. A. Vorp, and S. Maiti. A mechanistic model on the role of “radially-running” collagen fibers on dissection properties of human ascending thoracic aorta. J. Biomech. 47(5):981–988, 2014.
Rasband, W. S. ImageJ, U. S. National Institutes of Health, Bethesda, ML. http://imagej.nih.gov/ij/, 1997–2014.
Rosenberg, L. Chemical basis for the histological use of Safranin O in the study of articular cartilage. J. Bone Joint Surg. Am. 53:69–82, 1971.
Shah, S. B., C. Witzenburg, M. F. Hadi, H.P Wagner, J. M Goodrich, P. W Alford, and V. H. Barocas. Prefailure and failure mechanics of the porcine ascending thoracic aorta: experiments and a multiscale model. J. Biomech. Eng. 136(2):021028, 2014.
Sommer, G., T. C. Gasser, P. Regitnig, M. Auer, and G. A. Holzapfel. Dissection of the human aortic media: an experimental study. J. Biomech. Eng. 130:021007-1–021007-12, 2008.
Stemper, B. D., N. Yoganandan, and F. A. Pintar. Methodology to study intimal failure mechanics in human internal carotid arteries. J. Biomech. 38:2491–2496, 2005.
Tong, J., G. Sommer, P. Regitnig, and G. A. Holzapfel. Dissection properties and mechanical strength of tissue components in human carotid bifurcations. Ann. Biomed. Eng. 39(6):1703–1719, 2011. doi:10.1007/s10439-011-0264-y
Walpole, R., R. Meyers, S. Meyers, and K. Ye. Probability and Statistics for Engineers and Scientists, 9th ed. Pearson Education, Upper Saddle River, NJ, pp. 737–738, 2012.
Yuan, Y. The study of microstructure and mechanical behavior of an elastic artery and a muscular artery under normal and simulated trauma conditions. Phd thesis, University of Maryland, Baltimore County, 2007.
Acknowledgments
The experiments were performed in the University of Maryland, Orthopaedic Mechanobiology Laboratory, Dr. Adam H. Hsieh, Director. Portions of this work were presented from the podium at the Seventh World Congress of Biomechanics, Boston, MA, 2014.
Conflicts of interest
Henry W. Haslach, Jr., Lauren N. Leahy, Parinaz Fathi, Joshua M. Barrett, Amanda E. Heyes, Thomas A. Dumsha, and Eileen L. McMahon declare that they have no conflict of interest.
Human and animal rights and informed consent
No human studies were carried out by the authors for this article. No animal studies requiring institutional committee approval were carried out by the authors for this article because the bovine aortas were obtained from a slaughterhouse.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Haslach, H.W., Leahy, L.N., Fathi, P. et al. Crack Propagation and Its Shear Mechanisms in the Bovine Descending Aorta. Cardiovasc Eng Tech 6, 501–518 (2015). https://doi.org/10.1007/s13239-015-0245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-015-0245-7