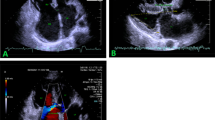
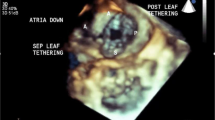
Abstract
Within this report we present a state of the art assessment of the status of 3D echocardiographic imaging (3D echo) of atrial septal defect (ASD) and ventricular septal defect (VSD). After a brief literature review of past studies, we delve into more recent developments in greater detail. For ASD we focus on new developments and reports regarding real-time transesophageal echocardiography. For VSD there have been no recent reports in the last 2 years dealing with new advances in 3D echo of VSD by either transthoracic or transesophageal approaches. Therefore, we present our experience in 3D echo of VSD by both methods. The literature is far from replete on this subject; therefore, a significant portion of this report is based on the authors’ personal experience using 3D echo in over 200 cases with ASD, VSD, or both lesions in children and adults. A collection of representative reference 3D echoes of ASD and VSD are presented to serve as a review of the anatomic variations which the echocardiographer and sonographer are likely to encounter.


















Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rivera JM, Siu SC, Handschumacher MD, et al. Three-dimensional reconstruction of ventricular septal defects: validation studies and in vivo feasibility. J Am Coll Cardiol. 1994;23:201–8.
Lang RM, Mor-Avi V, Sugeng L, et al. Three-dimensional echocardiography: the benefits of the additional dimension. J Am Coll Cardiol. 2006;48:2053–69.
Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric council of the American society of echocardiography. J Am Soc Echocardiogr. 2006;19:1413–30.
Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900.
Marx GR, Fulton DR, Pandian NG, et al. Delineation of site, relative size and dynamic geometry of atrial septal defects by real-time three-dimensional echocardiography. J Am Coll Cardiol. 1995;2:482–90.
Magni G, Cao QL, Sugeng L, et al. Volume-rendered, three-dimensional echocardiographic determination of the size, shape, and position of atrial septal defects: validation in an in vitro model. Am Heart J. 1996;132:376–81.
Acar P, Saliba Z, Bonhoeffer P, et al. Influence of atrial sepal defect anatomy in patient selection and assessment of closure with the cardioseal device. Eur Heart J. 2000;21:573–81.
Cao QL, Radtke W, Berger F, et al. Transcatheter closure of multiple atrial septal defects. Eur Heart J. 2000;21:941–7.
Van den Bosch AE, Harkel DJT, McGhie JS, et al. Characterization of atrial septal defect assessed by real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2006;19:815–21.
• Taniguchi M, Akagi T, Watanabe N, Okamoto Y, Nakagawa K, Kijima Y, Toh N, Ohtsuki S, Kusano K, Sano S. Application of real-time three-dimensional transesophageal echocardiography using a matrix array probe for trans-catheter closure of atrial septal defect. J Am Soc Echocardiogr 2009;22:1114–20. This article is the first detailed report of 3D TEE during ASD device closure.
•• Faletra FF, Ho SY, Auricchio A. Anatomy of right atrial structures by real-time 3D transesophageal echocardiography J Am Coll Cardiol Img. 2010;3:966–975. This report describes and illustrates in detail a 3D TEE imaging protocol and the anatomy of the right atrium and atrial septum with matched pathology specimens.
•• Pushparajah K, Owen I. Miller OI, Simpson JM. 3D Echocardiography of the atrial septum: anatomical features and landmarks for the echocardiographer. J Am Coll Cardiol Img. 2010;3:981–984. This article includes a fine collection of images of the normal atrial septum and atrial septal defects.
•• Saric M, Perk G, Purgess JR, Kronzon I. Imaging atrial septal defects by real-time three-dimensional transesophageal echocardiography: step-by-step approach. J Am Soc Echocardiogr 2010;23:1128–35. This report describes a detailed acquisition and imaging protocol for the 3D TEE assessment of ASD.
•• Roberson DA, Cui W, Patel D, et al. Three-Dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study J Am Soc Echocardiogr 2011;24:in press. This report provides a complete collection of the anatomic types of ASD as seen by 3D TEE.
Salustri A, Spitaels S, McGhie J, et al. Transthoracic three-dimensional echocardiography in adult patients with congenital heart disease. J Am Coll Cardiol. 1995;26:759–67.
Kardon RE, Cao QL, Masani N, et al. New insights and observations in three-dimensional echocardiographic visualization of ventricular septal defects. Circulation. 1998;98:1307–14.
Dall’Agata A, Cromme-Dijkhuis AH, Meijboom FJ, et al. Three-dimensional echocardiography enhances the assessment of ventricular septal defect. Am J Cardiol. 1999;83:1576–9.
Acar P, Abdel-Massih T, Douste-Blazy MY, et al. Assessment of muscular ventricular septal defect closure by transcatheter or surgical approach: a three-dimensional echocardiographic study. Eur J Echocardiogr. 2002;3:185–91.
Puri T, Liu Z, Doddamani S, et al. Three-dimensional echocardiography of post myocardial infarction cardiac rupture. Echocardiography. 2004;21:279–84.
Cheng TO, Xie MX, Wang XF, et al. Real-time 3-dimensional echocardiography in assessing atrial and ventricular septal defects: an echocardiographic-surgical correlative study. Am Heart J. 2004;148:1091–5.
Mehmood F, Miller AP, Nanda NC, et al. Usefulness of live/real time three-dimensional transthoracic echocardiography in the characterization of ventricular septal defects in adults. Echocardiography. 2006;23:421–7.
Chen FL, Hsiung MC, Nanda N, et al. Real time three-dimensional echocardiography in assessing ventricular septal defects: an echocardiographic-surgical correlative study. Echocardiography. 2006;23:562–8.
Van den Bosch AE, Ten Harkel DJ, McGhie JS, et al. Feasibility and accuracy of real-time 3-dimensional echocardiographic assessment of ventricular septal defects. J Am Soc Echocardiogr. 2006;19:7–13.
Mercer-Rosa L, Seliem MA, Fedec A, et al. Illustration of the additional value of real-time 3-dimensional echocardiography to conventional transthoracic and transesophageal 2-dimensional echocardiography in imaging muscular ventricular septal defects: does this have any impact on individual patient treatment? J Am Soc Echocardiogr. 2006;19:1511–9.
Seliem MA, Fedec A, Cohen MS, et al. Real-time 3-dimensional echocardiographic imaging of congenital heart disease using matrix-array technology: freehand real-time scanning adds instant morphologic details not well delineated by conventional 2-dimensional imaging. J Am Soc Echocardiogr. 2006;19:121–9.
Hsu JH, Wu JR, Dai ZK, Lee MH. Real-time three-dimensional echocardiography provides novel and useful anatomic insights of perimembranous ventricular septal aneurysm. Int J Cardiol. 2007;118:326–31.
Xiong Y, Wah YM, Chen M, et al. Real-time three-dimensional echocardiography using a matrix probe with live X-plane imaging of the interventricular septum. Ultrasound Obstet Gynecol. 2009;34:534–7.
Ishii M, Hashino K, Eto G, et al. Quantitative assessment of severity of ventricular septal defect by three dimensional reconstruction of color Doppler-imaged vena contracta and flow convergence region. Circulation. 2001;103:664–9.
Vasilyev NV, Melnychenko I, Kitahori K, et al. Beating-heart patch closure of muscular ventricular septal defects under real-time three-dimensional echocardiographic guidance: a preclinical study. J Thorac Cardiovasc Surg. 2008;135:603–9.
Hisagi M, Suematsu Y, Masuzawa A, et al. Image-guided surgical repair of ventricular septal rupture using self-expanding device. Interact Cardiovasc Thorac Surg. 2009;8:602–5.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roberson, D.A., Cui, V.W. Evaluation of Atrial and Ventricular Septal Defects with Real-Time Three-Dimensional Echocardiography: Current Status and Literature Review. Curr Cardiovasc Imaging Rep 4, 349–360 (2011). https://doi.org/10.1007/s12410-011-9102-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-011-9102-8