Abstract
Introduction
Patients with gout have numerous comorbidities. We aimed to estimate the prevalence and incidence rates of renal and cardiovascular morbidities in trial-aligned patients with established gout in Germany (DE), the United Kingdom (UK), the United States (US), and France (FR).
Methods
This longitudinal cohort study used retrospective data from IMS Disease Analyzer™ (DE, FR), Clinical Practice Research Datalink–Hospital Episode Statistics (UK), and IMS’ PharMetrics Plus database linked with outpatient laboratory results (US). Included patients were ≥18 years at index date (January 1, 2010; all dates +1 year for FR), with continuous enrollment during the pre-index year, had “prevalent established gout” determined by data in the pre-index year, and ≥1 documented visit after index date; additional inclusion/exclusion criteria were aligned with recent gout clinical trials. Look-back for comorbidity prevalence extended to January 1, 2003 (US: January 1, 2009). Follow-up for incidence extended from index date to at most March 26, 2013 (FR: May 31, 2014). Events of interest were identified by diagnostic codes and/or laboratory data.
Results
The trial-aligned cohorts included 35,118 (DE), 24,607 (UK), 121,591 (US), and 17,338 (FR) patients. Among renal conditions, baseline diagnosis of chronic kidney disease/renal failure was most prevalent in the UK followed by DE; abnormal serum creatinine was most prevalent in the UK. Hypertension was the most prevalent cardiovascular diagnosis in all countries, followed by ischemic heart disease (IHD) and myocardial infarction. Incidence rates (per 100 patient-years) for new/worsening renal impairment ranged from 1.67 (DE) to 4.34 (US) and for nephrolithiasis diagnosis from 0.31 (FR) to 3.79 (US). The incidence rates for hypertension diagnosis were highest among cardiovascular-related events, ranging from 3.23 (UK) to 20.27 (US), followed by IHD.
Conclusions
Patients with established gout such as those included in gout trials have a high burden of established morbidity and new diagnoses of morbid events. Consideration of comorbidities, which greatly exacerbate disease burden, is important in gout management.
Funding
AstraZeneca.
Similar content being viewed by others
Introduction
Gout is a urate crystal deposition disease that, when uncontrolled, is characterized by recurrent attacks (gout flares) of acute inflammatory arthritis of the peripheral joints. Continued urate crystal deposition due to uncontrolled disease can also lead to painful and disfiguring tophi, kidney stones, and uric acid nephropathy. Gout is the most common type of inflammatory arthritis in men and postmenopausal women, affecting 1–4% of the Western developed population [1, 2]. Gout is a chronic, progressively degenerative disease, and even when the patient is not experiencing flares or other signs and symptoms, the disease is ongoing and worsening. If not appropriately treated, gout can cause permanent joint destruction, bone erosion, and kidney damage [3, 4].
Risk factors for gout include dietary contributors, alcohol consumption, use of thiazide diuretics, and metabolic-related diseases (e.g., obesity, arterial hypertension, diabetes, hypercholesterolemia, coronary artery disease, congestive heart failure, and renal failure), as well as a genetic component, and in women, menopause [5]. The hallmark precursor to gout is hyperuricemia (serum uric acid [sUA] levels of >6.8 mg/dL [>400 μmol/L], i.e., the precipitation concentration for urate crystals) [6]. Hyperuricemia leads to the deposition of monosodium urate crystals (MSU) in musculoskeletal structures including joints, in kidneys, and in other connective tissue [7]. While diet and overproduction of uric acid can contribute (10%), the predominant cause of hyperuricemia is inefficient uric acid excretion (90%) [8]. A proportion of individuals may have both an overproduction and an inefficient excretion of uric acid [9].
In most patients, gout cannot be effectively treated by lifestyle changes alone. For long-term pharmacologic management of gout, the treatment guidelines from the European League against Rheumatism [4], the British Society of Rheumatology [10], and the American College of Rheumatology (ACR) [11] recommend treatments aimed at decreasing sUA for patients with recurrent acute attacks, tophi, or radiographic gout changes. Effective urate-lowering treatment (ULT) maintains uric acid below the critical level, prevents further MSU crystal formation, and over time dissolves away existing crystals [4]. The 2012 ACR Guidelines suggest xanthine oxidase inhibitor therapy with allopurinol or febuxostat as the first-line pharmacologic approach. In case first-line therapies do not succeed in reaching sUA targets or are contraindicated, uricosuric agents that increase renal excretion of uric acid (such as probenecid, sulfinpyrazone, benzbromarone, isobromindione) can be used [12]. Lesinurad increases urinary uric acid excretion and was recently approved in the US and EU to be used in combination with a xanthine oxidase inhibitor in patients unable to achieve target sUA on a xanthine oxidase inhibitor alone [13–15].
Hyperuricemia and gout are associated with comorbidities such as cardiovascular disease, chronic kidney disease (CKD), and metabolic syndrome including its components diabetes and hypertension [5, 16–24]. Patients with frequent gout attacks have higher prevalence of comorbid diseases, (e.g., CKD, hypertension, dyslipidemia, ischemic heart disease (IHD), heart failure, and arthritis) than those with infrequent attacks [25]. In addition, previous research has identified that morbidities associated with gout are directly related to gout disease severity [26]. Treatment challenges arise when patients have comorbid conditions and are being treated with multiple medications; these challenges include drug–drug interactions with treatments for renal disease or hepatic impairment, as well as with statins for the treatment of hypercholesterolemia [27–29]. Studies show that many patients with gout do not achieve treatment goals with current therapies, and the majority continue to experience recurrent acute attacks, further joint damage, and other complications [30]. Understanding morbidity prevalence and incidence rates in patients with gout is important for benchmarking optimal approaches to gout management and selecting optimal treatments for individual patients.
Patients with gout commonly have multiple comorbidities, with subsequent comorbidities arising as a result of the disease; this should be taken into consideration both in clinical practice and when conducting clinical trials in these patients. In this study, we analyzed the prevalence and incidence rates of potential disease- or treatment-related renal and cardiovascular morbidities in patients with established gout from databases in four countries, with eligibility criteria aligned with recent gout clinical trial inclusion criteria [31, 32] to provide a more appropriate context or baseline for understanding clinical trial rates of morbidities. CLEAR 1 (NCT01510158) and CLEAR 2 (NCT01493531) were two placebo-controlled, phase 3 trials that investigated the efficacy and safety of lesinurad (a selective uric acid reabsorption inhibitor) in combination with allopurinol in gout patients having an inadequate response to standard-of-care allopurinol, and were used here to help define cohort exclusion criteria and standardize age/sex distributions for the database study. The current analysis comprises data from patients in Germany (DE), the United Kingdom (UK), the US, and France (FR).
Methods
Data Sources
This study uses a retrospective data analysis of patients with gout using health-care data extracted from electronic medical record and administrative claims databases. The following databases were used: DE—IMS Disease Analyzer™ (IMS Health, Danbury, CT, US) databases [33, 34]; UK—Clinical Practice Research Datalink (CPRD) Hospital Episode Statistics [18, 35]; US—IMS PharMetrics Plus database [36–38]; FR—IMS Disease Analyzer database [39, 40]. A more detailed description of these databases can be found in Table 1.
Study Design and Patients
Study Design
A longitudinal cohort study design was used to evaluate (1) established prevalent comorbidity and (2) prospective incident comorbidity, in patients with prevalent established gout and eligibility criteria aligned with recent gout clinical trials.
Observation Periods
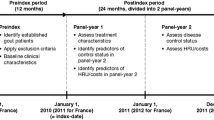
The overall study design is illustrated in Fig. 1. For the US, the UK and DE, the cohort baseline (index date) was January 1, 2010. For the FR analysis, the timeline was moved forward by 1 year (index date January 1, 2011) to synchronize with the supplementary information obtained by primary data collection (only available for 2012 and 2013, see details below). The 12-month period immediately preceding the index date was defined as the pre-index year and was used to identify eligible patients and determine most baseline characteristics. For determining some baseline comorbidities, the pre-index look-back was extended to January 1, 2003, in UK and DE (2004 in FR). The period following the index date was the follow-up period, and extended for each particular analysis to the first of the following occurrences in each patient: disenrollment, end of the study period, or an event of the outcome being studied in that particular analysis. The study end dates (i.e., last data available) were: DE: February 28, 2013; UK: March 26, 2013; US: December 31, 2012; FR: May 31, 2014.
To investigate morbidity potentially associated with current gout treatments, ULT-treated cohorts of patients treated with allopurinol, or with febuxostat, were also defined (Fig. 1). The first ULT treatment episode initiated after the main index date constituted the cohort entry criterion and defined the treatment episode index date. A treatment episode pre-index year and extended pre-index look-back were defined from the treatment episode index date. Follow-up for this cohort continued while on this first defined ULT treatment, i.e., until a switch or an add-on occurred or treatment disruption for a duration longer than the “admissible gap” (50% of the previous script’s duration), which terminated the first treatment episode, or otherwise until an event occurred, or until disenrollment, or the end of the study period. Dose change was not considered a treatment episode termination.
Patient Inclusion and Exclusion Criteria
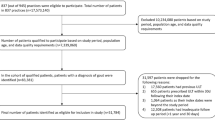
Since we aimed to investigate morbidity in a gout trial-aligned prevalent established gout cohort, inclusion criteria for the prospective cohort analysis included having prevalent established gout on the main index date (as assessed during the 1-year main pre-index period), being aged ≥18 years on the main index date, being continuously enrolled in the database during the pre-index year, and having at least one observation in the database after the index date (Fig. 2).
Established gout was defined as: at least one prescription of ULT documented during the pre-index year, or eligible for ULT according to ACR guidelines (i.e., a gout diagnosis documented during the pre-index year combined with either evidence of moderate CKD, diagnosis code for urolithiasis, diagnosis code for tophus, or occurrence of two gout flares).
Exclusion criteria included: at least one diagnosis during the pre-index year of hematological cancer, severe renal impairment, tumor lysis syndrome, Lesch–Nyhan syndrome or juvenile gout, missing data for important variables, or a payer type of “Medicare Cost” or “State Children’s Health Insurance Program” (US only) (Fig. 2). Additional exclusion criteria applied to identify patients meeting similar criteria to those used in recent gout clinical trials [31, 32] (“gout trial-aligned prevalent established gout”) included: history of myositis/myopathy or rhabdomyolysis; ≥1 prescription for systemic immunosuppressive/immunomodulatory treatment; treatment of active peptic ulcer disease within 4 weeks of index date; treatment with valproic acid or other known inhibitors before epoxide hydrolase within 90 days of the main index date; or ≥1 diagnosis code for HIV infection, hepatitis B or C infection, malignancy, unstable angina, heart failure, myocardial infarction, stroke, deep vein thrombosis, active liver disease, or hepatic dysfunction.
Morbidity
Identification of morbidity was primarily based on diagnostic codes at one inpatient (hospitalization) or outpatient visit (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] for US, Read/ICD-10 for UK, ICD-10 for FR and DE) and/or laboratory data (≥1 laboratory result during time period of interest), depending on the outcome of interest.
Morbidities of interest included CKD/renal impairment (pre-index for prevalence: diagnosis-based ≥1 diagnosis of CKD ≥Stage 2 or acute renal failure, or laboratory-based ≥1 elevated serum creatinine [sCr]; post-index for incidence: diagnosis-based new or worsening renal impairment identified through diagnosis codes, or laboratory-based relative increase in sCr of ≥1.5× over baseline), nephrolithiasis, cardiovascular conditions (essential hypertension, myocardial infarction, heart failure, IHD, pulmonary embolism, deep vein thrombosis), and diabetes. For FR, complementary observational data on hospitalizations and selected laboratory data (otherwise unavailable in the database) were collected retrospectively for a subset of the patients extracted from the database, based on voluntary participation by the general practitioners (GPs). Seventy-nine GPs participated, providing additional information on 943 patients. For FR, inpatient hospitalization data were thus unavailable for most patients and therefore outcomes that often require hospitalization may be underestimated for FR.
Statistical Analyses
Analyses are presented for the overall gout trial-aligned prevalent established gout cohort and for morbidity analyses also by first ULT treatment (allopurinol, febuxostat) in the period following the main index date.
For baseline demographics and patient characteristics, categorical measures are presented as frequency (number of cases) and percentage of the total study population observed. For continuous variables, descriptive statistics are presented.
To obtain rates relevant for gout clinical trial context, the prevalence and incidence rates were standardized to the approximate age and sex distribution of a gout trial population [31, 32] to account for the difference in age and sex distributions between such a prevalent established gout trial population and the observational gout trial-aligned prevalent established gout cohorts in this study. Crude observational cohort rates are also presented in Supplementary Tables.
To describe the baseline comorbidity of the cohorts, frequency (number of cases) and prevalence rates (percentage) standardized to the age and sex distribution of gout clinical trial patients are presented, of patients with ≥1 event (diagnosis and/or laboratory results) for each respective condition documented at any time in the 1 year pre-index in the US, and for pulmonary embolism and deep vein thrombosis in FR, DE, UK, or from January 1, 2003 (2004 in FR) for the remaining morbid events in FR, DE, and UK, until the main index date (or treatment-related index date, respectively).
The incidence of morbidities related to laboratory values were assessed only in the subgroup of subjects with available laboratory data (i.e., subjects with linkable laboratory values). For the US, laboratory data were obtained for an external linkable source and were available for a subsample of 8% of the patients with gout used for this study. For the UK and DE, all laboratory tests were in principle available for all subjects, so the absence of a test indicates it was not performed. For FR, laboratory tests for the outcomes studied were not systematically available (see details above).
Age- and sex-standardized incidence rates for each morbidity event are presented per 100 person-years (PY), with 95% confidence intervals. Follow-up for each event analysis started from the index date and ended at the occurrence of the first event (for patients with an event), death, disenrollment from the health plan (for subjects not experiencing the event), or the study end date.
Hypertension, heart failure, IHD, and diabetes were considered as chronic conditions, while all other studied morbidities were considered as acute. Patients with prevalent chronic morbidities at index date were excluded from the analysis of incidence post-index of these morbidities. The incidence of the acute conditions in the post-index period was evaluated independent of their occurrence in the pre-index period.
Data were analyzed using SAS software version 9 (SAS Institute, Cary, NC, USA).
Compliance with Ethics Guidelines
This article is based primarily on previously and routinely collected data in the databases used for the study, in compliance with the rules for each database. The UK part of this study was approved by the Independent Scientific Advisory Committee for MHRA database research (ISAC) under protocol number 13_134, as required for use of CPRD data. Some complementary retrospective data were collected in France from a sample of general practitioners participating in the French Disease Analyzer database, with approval obtained from the “CNIL” (“Commission Nationale de l’Informatique et des Libertés”, ref: MMS/MKE/AR/144351). Beyond this, the current report does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Baseline Characteristics
In total, 71,622 (DE), 44,775 (UK), 313,311 (US), and 29,645 (FR) patients were identified with prevalent established gout as defined during the main pre-index year. After applying trial-aligned inclusion and exclusion criteria, the gout trial-aligned prevalent established gout cohorts comprised a total of 35,118 (DE), 24,607 (UK), 121,591 (US), and 17,338 (FR) patients (Fig. 2).
The characteristics of the gout trial-aligned prevalent established gout cohort at baseline, on the index date, are shown in Table 2. Patients from DE, the UK, and FR were similar with regard to age and body mass index (BMI), while patients from the US generally were younger. BMI was unavailable for US patients, and only available for a minority of patients in the other countries. Patients from the UK had the highest mean number of days on ULT during the 1-year pre-index period.
Several studied groups of co-medications of interest with respect to the outcomes studied were found to be common in the pre-index year (Table 2) [41]. Approximately, 22–38% of patients across countries were treated with diuretics and angiotensin-converting enzyme inhibitors, respectively. Drugs considered to potentially be associated with sUA decrease were taken by a large majority, 63–82%, and almost all patients were taking some drug with potential nephrotoxic effects.
Prior to the index date, a recorded diagnosis of essential hypertension was most prevalent in patients from DE and least prevalent in patients from the UK (Table 2). Obesity was most prevalent in patients from the UK and diabetes was most prevalent in patients from DE.
Standardized Prevalence Rates of Morbid Events
Standardized prevalence rates of morbidity events potentially associated with gout or gout treatment are presented in Table 3; for corresponding crude rates, see Table S2 in the supplementary materials.
Among renal conditions, diagnosis of CKD/renal failure was most prevalent in the UK (10.9%) followed by DE (6.7%); prevalence of abnormal sCr was also highest in the UK (30.6%). Hypertension was the most prevalent cardiovascular diagnosis in all four countries, followed by IHD and myocardial infarction.
Standardized Incidence Rates of Morbid Events
Standardized incidence rates (per 100 PY) of diagnosis-based morbidity events potentially associated with gout or gout treatment in the gout trial-aligned prevalent gout cohort are presented in Table 4; for corresponding crude rates, see Table S3 in the supplementary materials.
Estimated standardized incidence rates of new/worsening renal impairment ranged from 1.67 (DE, overall group) to 21.72 (US, febuxostat-treated), with that of nephrolithiasis diagnosis ranging from 0.12 (FR, overall group) to 5.65 (US, allopurinol-treated). The incidence rate of hypertension diagnosis was highest among cardiovascular-related rates in all groups, ranging from 3.23 (UK, overall group) to 39.46 (US allopurinol-treated), followed by IHD. Relatively low numbers of patients in the febuxostat cohort resulted in wide confidence intervals (Table 4).
For laboratory-based renal impairment, the incidence rates of sCr elevations of ≥1.5× over the pre-index baseline in these data with likely underreporting were 0.49 (FR), 0.71 (US), and 0.84 (UK, DE) in the overall cohort, in the subgroups with available laboratory data.
Discussion
Patients with gout frequently have multiple comorbidities, including hypertension, CKD, cardiovascular disease, obesity, diabetes, and hyperlipidemia, all of which have a significant adverse impact on public health [25, 42]. In some cases (e.g., CKD), the presence of the comorbidity contributes to the progression of hyperuricemia and/or gout [43]. Whether gout or hyperuricemia themselves contribute to the pathogenesis of gout comorbidities is an area of intensifying investigation.
The results presented herein confirm that there is a notable degree of comorbidity burden among patients with gout. In agreement with other studies [26, 44], hypertension was the most prevalent cardiovascular diagnosis in gout patients, but there was also a high percentage of patients from each country with hyperlipidemia and/or diabetes. The clinical characteristics of the patients in this study were similar to those described in another recent large US population-based study of 36,431 patients with chronic gout [42] treated with allopurinol, febuxostat, or colchicine in which more than half had hypertension and hyperlipidemia, 19–23% had diabetes, and 9–12% had cardiovascular disease. As gout is known to increase the risk of mortality from cardiovascular disease and coronary heart disease [18], and higher cardiovascular risk is associated with the severity of gout [45], it is important to correctly identify and manage cardiovascular risk factors in patients with gout, and also to identify and optimally manage gout in patients at risk of cardiovascular disease. The findings from this study also demonstrate that patients with gout are subsequently diagnosed with other morbidity events.
Conclusions regarding differences between the two ULT treatment cohorts are difficult to draw due to the relatively low numbers of patients in the febuxostat cohort. A previous study reported that CKD was twice as common in febuxostat initiators compared with allopurinol or colchicine initiators [42] and that febuxostat initiators had more comorbid conditions, greater use of medications and health care resources compared with the other groups. This may be due to the fact that febuxostat is generally used as the second-line treatment after allopurinol and therefore patients are more likely to have more severe disease and/or comorbidities than those receiving first-line treatment. In our study, we noted numerically higher standardized CKD/renal prevalence in the febuxostat than in the allopurinol cohort as the only consistent difference across countries for prevalence rates, while there was no consistent difference between the treatments for incidence of new/worsening renal impairment.
The associations between gout and occurrence of several comorbidities are well established [21]. For example, the association between gout and the components of metabolic syndrome can be explained, in part, through effects on urate production, renal angiotensin secretion, and renal urate excretion [46, 47]. Gout is also a known risk factor for cardiovascular diseases such as cerebrovascular disease, congestive heart failure, and myocardial infarction [48–51], collectively leading to an increased risk for all-cause and cardiovascular mortality in patients with gout [52]. A recent study of a large UK primary care database of patients with gout and matched controls found that risks for incident comorbidity were higher not only for cardiovascular, metabolic/endocrine, and musculoskeletal diseases, but also for genitourinary disease, liver diseases, hemiplegia, depression, anemia, and psoriasis [21].
In our study, we saw relatively high incidence rates of several renal and cardiovascular comorbidities. Using four different databases provided a range of estimates for relevant incidence and prevalence of comorbidities. Each database had distinct strengths and limitations and used different resources in different countries and each estimate needs to be interpreted with caution. Some databases may have captured some comorbidities better than others. Several limitations to this study deserve discussion. First, there was variability across the four countries in prevalent comorbidity and new incident diagnostic and/or laboratory events. This could be related to true differences in the populations, differences in disease course or treatment intensity between countries, and also to methodological and other differences in the databases analyzed. Since we mainly relied on the diagnosis codes and laboratory-specific data to select patients with gout and identify their comorbidities, potential misclassification bias is as always a caveat and may be different across the databases. In addition, the prevalence rates were assessed in a “gout trial-aligned” cohort of patients with prevalent established gout, meaning that subjects with some specific comorbidities of interest (cardiovascular diseases, liver conditions, severe CKD) documented 1 year prior to the main index date had been excluded from the analysis, which likely resulted in somewhat lower prevalence rates and, to a more limited extent, decreased incidence rates of some morbidity conditions.
Several country-specific limitations to the study due to the nature of the individual databases used should also be discussed. In DE, the percentage of physicians represented (2.4%) was not a random sample and was small. As a consequence, the patients sampled might not be representative of the whole German population. For the UK data, the prevalence rates may potentially be somewhat underestimated, particularly with chronic conditions, as an artifact of the CPRD database where patients remain linked to the same practice for a long time and chronic conditions may not be documented repeatedly; to some extent, this was likely alleviated by the relatively extended look-back period to 2003 for many pre-index comorbidities. The US PharMetrics Plus database is representative of commercially insured working adults, so generalizability to older populations (aged ≥65 years) is limited. In addition, laboratory data were not directly available in the database and were acquired through a third-party vendor, thereby limiting identification of laboratory values for all patients in the study and resulting in outcomes based on laboratory values that may not be representative of the whole population. In general, claims data have inherent limitations as they are collected for billing and reimbursement purposes rather than for research purposes. Abnormal laboratory data are likely underestimated in all databases, since all patients are not tested, and absence of an abnormal value cannot automatically be assumed to imply a normal value. In FR, as in the US, due to the limitations of availability of laboratory data for all patients and the nature of the claims database, some underreporting of many incidence rates is likely. In addition, some patients with prevalent gout may self-treat, and as a result may not always show up in health databases. Some outcomes that are often hospitalized may also be underreported where hospitalizations are not completely captured, e.g., in FR, and in DE where only referrals but not emergency hospitalizations are captured in the database. More generally, the estimated prevalence rates would be expected to increase when increasing the look-back period. This is supported by the UK data, which show that the crude prevalence of hypertension, which was 32.4% when assessed with 7 years look-back to January 1, 2003 (Table 2), was 5.7% when assessed only over the 1-year pre-index period. Specificity of the outcome definition will also affect the prevalence estimates, for example the estimated crude prevalence of hypertension in the UK data dropped from 32.4% to 4.6% when requiring two outpatient visits rather than only one for the condition. Overall, the retrospective observational nature of this study, its use of secondary data, and the analytical definitions used should be considered when interpreting the results.
Conclusions
In summary, it is difficult to quantify an “exact” burden based on these data; nevertheless, this study fills an important current gap by providing a range of potentially relevant estimates of prevalence and incidence of these conditions, obtained using as consistent methodology as possible across databases, in a large real-world setting reflective of today’s patient populations in these four countries. As a whole, our findings confirm that there is a notable degree of renal and cardiovascular comorbidity burden among patients with gout. Additionally, the results demonstrate that patients with gout, followed over time, continue to be frequently diagnosed with new significant morbidity events.
References
Cea Soriano L, Rothenbacher D, Choi HK, Garcia Rodriguez LA. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther. 2011;13(2):R39.
Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–28.
Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1–4.
Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–24.
Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013;14:164.
Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep. 2014;16(2):400.
Perez-Ruiz F, Dalbeth N, Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv Ther. 2015;32(1):31–41.
Boss GR, Seegmiller JE. Hyperuricemia and gout. Classification, complications and management. N Engl J Med. 1979;300(26):1459–68.
Kannangara DR, Ramasamy SN, Indraratna PL, et al. Fractional clearance of urate: validation of measurement in spot-urine samples in healthy subjects and gouty patients. Arthritis Res Ther. 2012;14(4):R189.
Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372–4.
Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431–46.
Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447–61.
European Medicines Agency. European public assessment report (EPAR) for Zurampic. EMA Web site. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003932/human_med_001963.jsp&mid=WC0b01ac058001d124. Last accessed May 5, 2016.
Shen Z, Rowlings C, Kerr B, et al. Pharmacokinetics, pharmacodynamics, and safety of lesinurad, a selective uric acid reabsorption inhibitor, in healthy adult males. Drug Des Devel Ther. 2015;9:3423–34.
US Food and Drug Administration. FDA approves Zurampic to treat high blood uric acid levels associated with gout. U S FDA Web site. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm478791.htm. Last accessed May 5, 2016.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–15.
Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120(5):442–7.
Clarson LE, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK Clinical Practice Research Datalink. Ann Rheum Dis. 2015;74(4):642–7.
Jing J, Kielstein JT, Schultheiss UT, et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant. 2014;30(4):613–21.
Juraschek SP, Kovell LC, Miller ER III, Gelber AC. Association of kidney disease with prevalent gout in the United States in 1988–1994 and 2007–2010. Semin Arthritis Rheum. 2013;42(6):551–61.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–7.
Lopez-Suarez A, Elvira-Gonzalez J, Bascunana-Quirell A, et al. Serum urate levels and urinary uric acid excretion in subjects with metabolic syndrome. Med Clin (Barc). 2006;126(9):321–4.
Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–31.
Puig JG, Martinez MA. Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol. 2008;20(2):187–91.
Saseen JJ, Agashivala N, Allen RR, Ghushchyan V, Yadao AM, Nair KV. Comparison of patient characteristics and gout-related health-care resource utilization and costs in patients with frequent versus infrequent gouty arthritis attacks. Rheumatology (Oxford). 2012;51(11):2004–12.
Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011;12:103.
Knake C, Stamp L, Bahn A. Molecular mechanism of an adverse drug-drug interaction of allopurinol and furosemide in gout treatment. Biochem Biophys Res Commun. 2014;452(1):157–62.
Richette P, Frazier A, Bardin T. Pharmacokinetics considerations for gout treatments. Expert Opin Drug Metab Toxicol. 2014;10(7):949–57.
Ryu HJ, Song R, Kim HW, et al. Clinical risk factors for adverse events in allopurinol users. J Clin Pharmacol. 2013;53(2):211–6.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case–control study. Ann Rheum Dis. 2014;75(1):210–7.
Bardin T, Keenan R, Khanna P, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with allopurinol: results from a phase III study in gout patients having an inadequate response to standard of care (CLEAR 2). Ann Rheum Dis. 2015;74(Suppl 2):545 (Abstract FRI0333).
Saag K, Fitz-Patrick D, Kopicko J, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with allopurinol: results from a phase III study in gout patients having an inadequate response to standard of care (CLEAR 1). Ann Rheum Dis. 2015;74(Suppl 2):540 (Abstract FRI0320).
Kostev K, Dippel FW, Bierwirth R. Resource consumption and costs of treatment in patients with type 1 diabetes under intensified conventional therapy under German real-life conditions. J Diabetes Sci Technol. 2013;7(3):736–42.
Rathmann W, Kostev K, Gruenberger JB, Dworak M, Bader G, Giani G. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013;15(1):55–61.
Gallagher AM, van Staa TP, Murray-Thomas T, et al. Population-based cohort study of warfarin-treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open. 2014;4(1):e003839.
Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ. 2014;17(10):696–707.
Bergvall N, Lahoz R, Reynolds T, Korn JR. Healthcare resource use and relapses with fingolimod versus natalizumab for treating multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin. 2014;30(8):1461–71.
Curtis JR, Schabert VF, Yeaw J, et al. Use of a validated algorithm to estimate the annual cost of effective biologic treatment for rheumatoid arthritis. J Med Econ. 2014;17(8):555–66.
Grandfils N, Detournay B, Attali C, et al. Glucose lowering therapeutic strategies for type 2 diabetic patients with chronic kidney disease in primary care setting in france: a cross-sectional study. Int J Endocrinol. 2013;2013:640632.
Misery L, Ansolabehere X, Grandfils N, Georgescu V, Taieb C. Nine-year follow-up of children with atopic dermatitis by general practitioners. Dermatology. 2014;228(4):344–9.
Daskalopoulou SS, Tzovaras V, Mikhailidis DP, Elisaf M. Effect on serum uric acid levels of drugs prescribed for indications other than treating hyperuricaemia. Curr Pharm Des. 2005;11(32):4161–75.
Kim SC, Schmidt BM, Franklin JM, Liu J, Solomon DH, Schneeweiss S. Clinical and health care use characteristics of patients newly starting allopurinol, febuxostat, and colchicine for the treatment of gout. Arthritis Care Res (Hoboken). 2013;65(12):2008–14.
Karis E, Crittenden DB, Pillinger MH. Hyperuricemia, gout, and related comorbidities: cause and effect on a two-way street. South Med J. 2014;107(4):235–41.
Riedel AA, Nelson M, Wallace K, Joseph-Ridge N, Cleary M, Fam AG. Prevalence of comorbid conditions and prescription medication use among patients with gout and hyperuricemia in a managed care setting. J Clin Rheumatol. 2004;10(6):308–14.
Perez-Ruiz F, Martinez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177–82.
Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998;8(4):250–61.
Vuorinen-Markkola H, Yki-Jarvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab. 1994;78(1):25–9.
Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol. 1988;41(3):237–42.
Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894–900.
Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688–96.
Kuo CF, Yu KH, See LC, et al. Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatology (Oxford). 2013;52(1):111–7.
Clarson L, Chandratre P, Hider S, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22(3):335–43.
Acknowledgments
Sponsorship, article processing charges, and the open access charge for this study were funded by AstraZeneca, Wilmington, DE, USA. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Editorial support was provided by Bill Wolvey of PAREXEL, Uxbridge, UK. Support for this assistance was funded by AstraZeneca.
Disclosures
Javier Nuevo is an employee of AstraZeneca. Fredrik Nyberg is an employee of AstraZeneca. Laura Horne is a former employee of AstraZeneca, currently a contractor to AstraZeneca. (During the conduct of this study, Laura Horne was an employee of AstraZeneca). Robert Morlock is an employee of Ardea Biosciences, a member of the AstraZeneca Group. Chris Storgard is an employee of Ardea Biosciences, a member of the AstraZeneca Group. Lalitha Aiyer is a former employee of Ardea Biosciences, a member of the AstraZeneca Group. (During the conduct of this study Lalitha Aiyer was an employee of Ardea Biosciences.) Dionne Hines is an employee of IMS Health. Xavier Ansolabehere is an employee of IMS Health. Pierre Chevalier is an employee of IMS Health.
Compliance with Ethics Guidelines
This article is based primarily on previously and routinely collected data in the databases used for the study, in compliance with the rules for each database. The UK part of this study was approved by the Independent Scientific Advisory Committee for MHRA database research (ISAC) under protocol number 13_134, as required for use of CPRD data. Some complementary retrospective data were collected in France from a sample of general practitioners participating in the French Disease Analyzer database, with approval obtained from the “CNIL” (“Commission Nationale de l’Informatique et des Libertés”, ref: MMS/MKE/AR/144351). Beyond this, the current report does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/62D4F0603A5B541A.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nyberg, F., Horne, L., Morlock, R. et al. Comorbidity Burden in Trial-Aligned Patients with Established Gout in Germany, UK, US, and France: a Retrospective Analysis. Adv Ther 33, 1180–1198 (2016). https://doi.org/10.1007/s12325-016-0346-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0346-1