Abstract
Introduction
We investigated the possibilities of drug–drug interactions between luseogliflozin, a sodium–glucose co-transporter-2 inhibitor, and oral antidiabetic drugs (OADs) in healthy Japanese males.
Methods
We conducted six independent studies to investigate potential drug–drug interactions between 5 mg luseogliflozin and the following OADs usually used in Japan: 1 mg glimepiride, 250 mg metformin, 30 mg pioglitazone, 50 mg sitagliptin, 50 mg miglitol, or 0.6 mg voglibose (0.2 mg before each meal). Twelve subjects were enrolled in each study. The glimepiride, metformin, sitagliptin, and miglitol studies were randomized, open-label, single-dose, three-way crossover studies. The pioglitazone and voglibose studies were open-label studies, where a single dose of luseogliflozin was added to multiple doses of pioglitazone or voglibose. The endpoints were the area under the curve from 0 to 24 h (AUC0–24h) or to infinity (AUCinf) and the maximum concentration (C max) of each drug administered alone or in combination.
Results
The 90% confidence intervals (CIs) of the geometric mean ratio (GMR) for C max of luseogliflozin in the pioglitazone and miglitol studies were beyond the reference range for bioequivalence (0.80–1.25) (miglitol: 0.851 [0.761, 0.952]; pioglitazone: 1.16 [1.04, 1.30]). However, the 90% CIs for AUC0–24h were within the reference range. The 90% CIs of the GMRs for C max and AUC0–24h of pioglitazone were beyond the reference range (C max 0.884 [0.746, 1.05]; AUC0–24h 0.896 [0.774, 1.04]), but the 90% CIs for the active metabolites of pioglitazone were within the reference range. For the other combinations tested, the 90% CIs and GMRs for luseogliflozin and the individual OADs were within the reference range.
Conclusion
No clinically meaningful interactions were observed between luseogliflozin and six commonly used OADs in Japan, although there were some changes in the pharmacokinetics of pioglitazone co-administered with luseogliflozin and for luseogliflozin co-administered with miglitol or pioglitazone.
Funding
Taisho Pharmaceutical Co., Ltd.
Similar content being viewed by others
Introduction
Most patients with type 2 diabetes mellitus (T2DM) have insufficient glycemic control on diet and exercise therapy, and require monotherapy with an oral antidiabetic drug (OAD) to improve glycemic control [1], which may be followed by a combination of two or more OADs [1]. Therefore, it is important to investigate the efficacy and safety of a variety of combinations of OADs in the clinical setting, especially with the introduction of novel classes of drugs, like sodium–glucose co-transporter (SGLT) 2 inhibitors.
Luseogliflozin is a potent and selective SGLT2 inhibitor [2, 3] that enhances urinary glucose excretion (UGE) by inhibiting glucose reuptake in the kidney and lowers plasma glucose concentrations in a dose-dependent manner [4]. It was recently approved in Japan [5] for the treatment of T2DM based on the results of Phase II and Phase III studies [6–8]. Considering the insulin-independent mechanism of action of SGLT2 inhibitors and because many patients require combination therapy, the clinical development program for luseogliflozin included testing its efficacy and safety in combination with other OADs. Before implementing large-scale clinical trials, it was necessary to conduct pharmacokinetic studies to assess the potential for drug–drug interactions when co-administering luseogliflozin with other OADs.
In accordance with the Japanese treatment guidelines [9], clinicians select the first OAD taking into account the patient’s status, and several types of OADs, including metformin, pioglitazone, insulin secretagogues (sulfonylureas and glinides), dipeptidyl peptidase 4 inhibitors, and α-glucosidase inhibitors, are often used for this purpose. Therefore, it is important to know whether SGLT2 inhibitors can be safely and effectively used in combination with other OADs in clinical settings.
In this report, we describe the results of six independent clinical trials that evaluated the potential for drug–drug interactions between luseogliflozin and glimepiride, metformin, pioglitazone, sitagliptin, miglitol, or voglibose. The results of these studies were used to inform the design of large-scale Phase III studies used to establish the indications and potential combinations of luseogliflozin with OADs in clinical practice.
Methods
Ethics Statement
The studies were conducted in accordance with the standards of the Japanese Pharmaceutical Affairs Law and Good Clinical Practice. The study protocols were approved by the Institutional Review Board of the participating study institutions. All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2000 and 2008. All the participants provided written informed consent.
Eligibility Criteria
Japanese healthy males aged 20–39 years and with a body mass index of 18.5–24.9 kg/m2 were eligible for the studies. All participants underwent screening tests (medical examination, vital signs, electrocardiograms and laboratory tests) to confirm their eligibility. Subjects were excluded from the studies if they had any clinically significant disease or disorder, at the investigator’s discretion; had a history of renal disorder, diabetes mellitus, or impaired glucose tolerance; had a serum creatinine concentration above the upper limit of the reference range in the study institutions; or tested positive for urinary protein or occult blood. Subjects were prohibited from using any drugs within 1 week of the first dose of the study drug. Twelve subjects were to be enrolled in each study.
Study Design
For this study, we opted to use 5 mg luseogliflozin because this was expected to be an effective clinical dose based on the results of an earlier Phase II study [6]. Six independent open-label studies were conducted to evaluate the potential for drug–drug interactions between luseogliflozin and representatives of the OADs often used in Japan (glimepiride, metformin, pioglitazone, sitagliptin, miglitol, and voglibose). Each study was conducted at a single medical institution. The glimepiride and metformin studies were conducted at Heishinkai Medical Group Incorporated OPHAC Hospital, Osaka, Japan, between August 2010 and September 2010. The pioglitazone and sitagliptin studies were conducted at Medical Co. LTA. Sumida Hospital, Tokyo, Japan, between September 2010 and October 2010. The voglibose study was conducted at Medical Co. LTA. Sumida Hospital between September 2010 and November 2010. The miglitol study was conducted at Heishinkai Medical Group Incorporated OCROM Clinic, Osaka, Japan, between January 2012 and March 2012. In all the studies, the participants were enrolled and allocated to the specified treatment by the investigators. There were no changes to the study designs, outcomes, or analyses after starting enrollment.
Glimepiride, Metformin, Sitagliptin, and Miglitol Studies
The drug–drug interactions between luseogliflozin and glimepiride, metformin, sitagliptin, and miglitol were examined in randomized, open-label, single-dose, three-way crossover studies. In each of these studies, the subjects received three treatments: one dose of luseogliflozin (A); one dose of the test OAD (B); and one dose of luseogliflozin plus the test OAD (C). The order of treatments was randomized based on six treatment sequences (ABC, ACB, BCA, BAC, CAB, CBA) with a washout period of ≥7 days between treatments. Subjects were hospitalized from 2 days before administration of the study drug to 1 day after administration of the study drug in each treatment period. The doses of the study drugs were 5 mg luseogliflozin, 1 mg glimepiride, 250 mg metformin, 50 mg sitagliptin, and 50 mg miglitol. The dose of each drug was selected based on the approved dose range for use in Japan and considering the safety and pharmacokinetic profiles of each drug in healthy Japanese males. Luseogliflozin and the specified OAD were administered before breakfast in the glimepiride, sitagliptin, and miglitol studies, and under fasting conditions in the metformin study. During hospitalization, the subjects were prohibited from consuming food/drink other than the meals provided by the clinic. Pharmacokinetic data sampling was performed for 24 h after drug administration. Venous blood samples were obtained for pharmacokinetic analyses before study drug administration and at 0.25, 0.5, 1, 1.5, 2, 2.5 (glimepiride and metformin studies only), 3, 4, 6, 8, 12, and 24 h after study drug administration in each treatment period. Pooled urine samples were obtained for 24 h before study drug administration and from 0 to 24 h after study drug administration in each treatment period to measure 24-h cumulative UGE.
Pioglitazone and Voglibose Studies
The potential for drug–drug interactions between luseogliflozin and pioglitazone or voglibose was examined in open-label, add-on studies. The add-on design was chosen after considering the time needed to reach the trough concentration of the unchanged compound and all active metabolites for pioglitazone in steady-state conditions, and considering the time needed for voglibose to reach peak exposure in the gastrointestinal tract for steady-state effects. In the pioglitazone study, the subjects received 5 mg luseogliflozin on Day 1, 30 mg pioglitazone on Days 2–7, and 5 mg luseogliflozin plus 30 mg pioglitazone on Day 8. The dose of each drug was selected based on the dose commonly used in Japan. All drugs were administered before breakfast. Pharmacokinetic data were collected on Day 1 (corresponding to luseogliflozin monotherapy), Day 7 (pioglitazone monotherapy), and Day 8 (combination therapy). The same procedure was used in the voglibose study, except the subjects received 0.6 mg voglibose (0.2 mg before each meal) on Days 2–7, and pharmacokinetic data were not collected for voglibose. In both studies, the subjects were hospitalized from 2 days before the first dose of the study drugs (Day −2) to Day 9. Venous blood samples were obtained for pharmacokinetic analyses before study drug administration and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after study drug administration in each study. Pooled urine samples were obtained for 24 h before study drug administration and from, 0 to 24 h after study drug administration on Days 1, 7 and 8 to measure 24-h cumulative UGE.
Pharmacokinetic Assessments
Blood samples used to determine the concentrations of the study drugs were collected at predetermined times selected based on the characteristics of each drug. The pharmacokinetic endpoints were the plasma concentrations of luseogliflozin and the OAD when administered alone or in combination. The plasma concentrations of the active metabolites of pioglitazone, M-III and M-IV, were also measured. Because voglibose is not orally absorbed, the plasma concentration of voglibose was not measured. Blood samples were immediately processed to plasma by centrifugation and were stored at −70 °C until analysis.
The plasma concentration of luseogliflozin was analyzed using a validated high-performance liquid chromatography tandem mass spectrometry (LC–MS/MS) method, as previously described [4]. The lower limit of quantification (LLOQ) and the upper limit of quantification (ULOQ) for the luseogliflozin plasma concentration were 0.05 and 100 ng/mL, respectively. The plasma concentrations of glimepiride, metformin, miglitol, and sitagliptin were determined by separate, validated LC–MS/MS methods with stable isotope-labeled internal standards (glimepiride-d5, metformin-d6, miglitol-d4, and sitagliptin-d4). The quantitative range (LLOQ to ULOQ) was 0.5–500 ng/mL for glimepiride, 5–2000 ng/mL for metformin, 5–3000 ng/mL for miglitol, and 1–500 ng/mL for sitagliptin. The plasma concentrations of pioglitazone and its active metabolites (M-III, keto derivatives of pioglitazone; and M-IV, hydroxy derivatives of pioglitazone) were simultaneously determined by validated LC–MS/MS methods with stable isotope-labeled internal standards (pioglitazone-d4, keto pioglitazone-d4, and hydroxy pioglitazone-d5). The quantitative range (LLOQ to ULOQ) was 10–2000 ng/mL for pioglitazone, M-III, and M-IV. All validation results met the predefined acceptance criteria. The analyses of luseogliflozin and the OADs were performed by JCL Bioassay Corp. (Nishiwaki, Japan).
We calculated the following pharmacokinetic parameters using the non-compartmental model: the maximum concentration (C max), the time to the maximum concentration (t max), and the area under the concentration–time curve from 0–24 h (AUC0–24h) or to infinity (AUCinf). The presence of drug–drug interactions was assessed based on the reference range for bioequivalence of 0.8–1.25 for geometric mean ratios (GMRs) with 90% confidence intervals (90% CIs) of the combination therapy relative to monotherapy, as recommended by the Japanese Pharmaceutical and Food Safety Bureau [10].
Other Assessments
To assess the effects of luseogliflozin, test OADs, and each combination on UGE, urine samples were collected at predetermined times and the urine glucose concentrations were measured. Safety assessments were based on the nature and frequency of adverse events, including changes in laboratory values, vital signs, and 12-lead electrocardiography. Hypoglycemia was assessed by each investigator considering symptoms or blood glucose levels in each subject.
Statistical Analyses
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) for the miglitol study, and SAS version 9.1.3 for the other studies. In the three-way crossover studies, the statistical significance of differences in C max, AUC0–24h and AUCinf were tested by analysis of variance (ANOVA) in which treatment, sequence, and timing were included as fixed effects and subject was included as a random effect. ANOVA was also used in the add-on studies using treatment as a fixed effect and subject as a random effect. Adverse events were coded using MedDRA version 14.1 for the miglitol study, and MedDRA version 13.1 for the other studies.
Results
Subjects
Twelve healthy males were enrolled in each study and all subjects completed the studies. The mean age, body weight, and body mass index in each study ranged from 24.3 to 26.8 years, from 60.25 to 65.68 kg, and from 20.45 to 21.97 kg/m2, respectively (Table 1).
Effects of Individual OADs on the Pharmacokinetics of Luseogliflozin
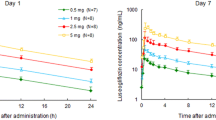
Figure 1 shows the plasma concentration–time profiles of luseogliflozin administered alone or in combination with the individual OADs. Table 2 shows the pharmacokinetic parameters of luseogliflozin with the GMRs. As indicated in Fig. 1, the plasma concentration–time profiles of luseogliflozin were similar when administered alone or in combination with the tested OADs.
When administered in combination with miglitol, the GMR (90% CI) for the C max of luseogliflozin was 0.851 (0.761, 0.952). Thus, the lower 90% CI was below the threshold for bioequivalence (0.80–1.25). By contrast, the GMRs and 90% CIs for AUC0–24h (0.969 [0.948, 0.991] and AUCinf (0.953 [0.931, 0.975]) were within the reference range. When administered in combination with pioglitazone, the GMR (90% CI) for the C max of luseogliflozin was 1.16 (1.04, 1.30). Therefore, the upper 90% CI was above the threshold for bioequivalence. The corresponding values for AUC0–24h (0.938 [0.901, 0.978]) and AUCinf (0.939 [0.897, 0.982]) were within the reference range. When administered in combination with glimepiride, metformin, sitagliptin and voglibose, the GMRs and 90% CIs for C max, AUC0–24h, and AUCinf of luseogliflozin were within the reference range.
Effects of Luseogliflozin on the Pharmacokinetics of Individual OADs
Figure 2 shows the plasma concentration–time profiles of each OAD alone or in combination with luseogliflozin. Table 3 shows the pharmacokinetic parameters of each OAD with the corresponding GMRs. The plasma concentrations of pioglitazone were slightly lower when co-administered with luseogliflozin than when pioglitazone was administered alone. By contrast, the plasma concentration–time profiles of the other OADs were similar when they were administered alone or in combination with luseogliflozin. Regarding pharmacokinetic parameters, the GMRs (90% CI) for C max and AUC0–24h of pioglitazone were 0.884 (0.746, 1.05) and 0.896 (0.774, 1.04), respectively. Therefore, the lower 90% CIs for both parameters were below the reference range for bioequivalence. However, the GMRs and 90% CIs for the active metabolites of pioglitazone (M-III and M-IV) were within the reference range for bioequivalence. When glimepiride, metformin, sitagliptin, and miglitol were administered with luseogliflozin, the GMRs and 90% CIs for C max, AUC0–24h, and AUCinf of each OAD were within the reference range for bioequivalence.
Plasma concentration–time profiles for glimepiride (a), metformin (b), pioglitazone (c), sitagliptin (d), and miglitol (e) administered alone or in combination with luseogliflozin. Curves are not shown for voglibose because it is not orally absorbed and its plasma concentrations were not measured. Values are presented as the means ± standard deviations
Urinary Glucose Excretion
As expected in healthy subjects, UGE was negligible in the presence of OADs alone. Administration of luseogliflozin in combination with an OAD slightly reduced UGE compared with luseogliflozin alone (Table 4).
Safety
There were no serious adverse events or adverse events requiring treatment discontinuation in any of the studies reported here. One adverse event (white blood cell urine positive) occurred in one subject treated with luseogliflozin in combination with glimepiride, and three events occurred in two subjects (nausea in one subject, nausea and headache in one subject) treated with luseogliflozin in combination with metformin. All these events were classified as mild in severity. There were no adverse events when luseogliflozin was administered alone or in combination with the other OADs. There were no episodes of hypoglycemia in any of the studies. In the glimepiride study, a decrease in the plasma glucose concentration occurred after administration of glimepiride, although there was no apparent difference between subjects treated with or without luseogliflozin. There were no clinically relevant changes in laboratory tests, vital signs, or electrocardiography.
Discussion
The results of the six independent studies reported here revealed no clinically relevant drug–drug interactions between luseogliflozin and six OADs used in Japan for the treatment of T2DM. Although there was a small change in the C max of luseogliflozin when co-administered with pioglitazone or miglitol, the changes in C max were small (about 15%), were not accompanied by changes in the AUC0–24h or AUCinf, and are unlikely to have a major influence on the pharmacokinetics of these drugs. Co-administration with luseogliflozin also resulted in a slight decrease in the C max and AUC0–24h for pioglitazone. However, the decreases were small (about 10%). The GMRs and 90% CIs for C max and AUC0–24h of its active metabolites, M-III and M-IV, which have slightly lower potency than pioglitazone [11], were within the reference range, and are also unlikely to have a major influence on the pharmacokinetics of these drugs or metabolites. When administered with the other OADs, the C max, AUC0–24h, and AUCinf of luseogliflozin and the OADs were within the reference range for bioequivalence.
Administration of luseogliflozin in combination with an OAD slightly reduced UGE compared with luseogliflozin alone. This might be expected considering the other OAD might elicit reductions in plasma glucose concentrations through increased uptake of glucose by the liver and other insulin-sensitive tissues or decreased absorption from the gastrointestinal tract in the case of voglibose.
The present results are consistent with those for other SGLT2 inhibitors, which also showed a lack of interactions with commonly used OADs [12–14]. This lack of interactions means SGLT2 inhibitors are well suited for use in combination with other commonly used OADs. Considering that SGLT2 inhibitors act in an insulin-independent manner, they can be used in combination with other drugs that target insulin secretion or insulin resistance. Although such combinations might increase the risk of hypoglycemia, the incidence of hypoglycemia is low in clinical trials, mostly because SGLT2 inhibitors normalize the renal glucose threshold, and UGE is reduced once the plasma glucose concentration reaches the renal glucose threshold [15]. Therefore, in clinical settings, the use of SGLT2 inhibitors in combination with another OAD is unlikely to increase the risk of hypoglycemia over the risk associated with the other OAD itself.
The cytochrome P450 (CYP) isoforms 3A4/5, 4A11, 4F2, and 4F3B, and UDP-glucuronosyltransferase 1A1 are involved in the metabolism of luseogliflozin (Taisho Pharmaceutical Co., Ltd., data on file). Because luseogliflozin is metabolized by multiple enzymes, administration of other drugs is unlikely to inhibit the metabolism of luseogliflozin or alter its pharmacokinetics. According to in vitro studies that evaluated the potential for inhibiting various CYP enzymes and organic anion transport proteins (OATPs), weak inhibition of CYP2C19 and OATP1B3 with half maximal inhibitory concentrations (IC50) of between 50 and 100 μmol/L were observed, and the IC50 values for other CYPs (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2D6, CYP2E1, and CYP3A4) and transporters (e.g., P-glycoprotein, breast cancer resistance protein, OATP1B1, OAT1, OAT3, and organic cation transporter 2) were consistently >100 μmol/L (Taisho Pharmaceutical Co., Ltd., data on file). Because the C max of 5 mg luseogliflozin in patients with T2DM was 0.69 μmol/L (299 ng/mL) (Taisho Pharmaceutical Co., Ltd., data on file), which is much lower than the concentration likely to inhibit CYP enzymes or transporters, luseogliflozin is unlikely to cause drug–drug interactions in relation to these enzymes and transporters. Furthermore, induction of CYP3A4 by luseogliflozin was observed at a concentration of 10 μmol/L in vitro. However, the 6β-hydroxycortisol/cortisol ratio, a marker for CYP3A4 induction [16], did not increase after 7 days of multiple doses of up to 25 mg of luseogliflozin in patients with T2DM in a clinical study conducted in the United States of America (Taisho Pharmaceutical Co., Ltd., data on file). Therefore, luseogliflozin is unlikely to induce CYP3A4 in clinical use.
In the present series of studies, luseogliflozin was well tolerated when used in combination with the OADs. There were no episodes of hypoglycemia when used in healthy subjects with normal glucose concentrations, even when used in combination with other OADs. Although urinary tract and genital infections occur in a small proportion of patients treated with SGLT2 inhibitors in clinical trials [7, 17, 18], this is unlikely to explain the presence of white blood cells in urine in one subject treated with single doses of luseogliflozin and glimepiride.
The absence of drug–drug interactions between luseogliflozin and the OADs in the present series of studies is consistent with the results of two long-term, Phase III studies [19]. These long-term studies demonstrated that luseogliflozin significantly reduced hemoglobin A1c and fasting plasma glucose when used in combination with other classes of OADs (biguanide, sulfonylurea, dipeptidyl peptidase 4 inhibitor, thiazolidinedione, glinide, and α-glucosidase inhibitor) for 52 weeks. Additionally, luseogliflozin was associated with a low rate of adverse drug reactions (12.4–25.4%). Hypoglycemia was most common in patients treated with luseogliflozin in combination with a sulfonylurea (10.7% over 52 weeks) and its incidence was lower when luseogliflozin was used in combination with other classes of OADs (0.9–3.4%), consistent with the mechanisms of action of each class of OAD.
Some limitations of the present studies warrant mention. In particular, the designs of the studies mean it is not possible to provide a comprehensive assessment of the safety or long-term efficacy of these combinations in patients with T2DM. Indeed, we did not assess the effects of the combinations used here on pharmacodynamic parameters or glycemic control. However, these were not the objectives of the present study, and several recently reported studies have shed light on the long-term efficacy and safety of luseogliflozin in combination with other OADs [19].
Conclusion
The results of this series of studies revealed no evidence of clinically significant drug–drug interactions between luseogliflozin and glimepiride, metformin, pioglitazone, sitagliptin, miglitol, or voglibose. These results suggest that luseogliflozin can be safely administered in combination with these OADs without a need for dose adjustments.
References
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79. doi:10.2337/dc12-0413.
Kakinuma H, Oi T, Hashimoto-Tsuchiya Y, Arai M, Kawakita Y, Fukasawa Y, et al. (1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-d-glucito l (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem. 2010;53:3247–61. doi:10.1021/jm901893x.
Yamamoto K, Uchida S, Kitano K, Fukuhara N, Okumura-Kitajima L, Gunji E, et al. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br J Pharmacol. 2011;164:181–91. doi:10.1111/j.1476-5381.2011.01340.x.
Sasaki T, Seino Y, Fukatsu A, Sakai S, Samukawa Y. Safety, pharmacokinetics, and pharmacodynamics of single and multiple luseogliflozin dosing in healthy Japanese males: a randomized, single-blind, placebo-controlled trial. Adv Ther. 2014;31:345–61. doi:10.1007/s12325-014-0102-3.
Markham A, Elkinson S. Luseogliflozin: first global approval. Drugs. 2014;74:945–50. doi:10.1007/s40265-014-0230-8.
Seino Y, Sasaki T, Fukatsu A, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, placebo-controlled, phase II study. Curr Med Res Opin. 2014;30:1219–30. doi:10.1185/03007995.2014.901943.
Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–55. doi:10.1185/03007995.2014.912983.
Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Dose-finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, double-blind, placebo-controlled, phase II study. Curr Med Res Opin. 2014;30:1231–44. doi:10.1185/03007995.2014.909390.
The Japan Diabetes Society. Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013. http://www.jds.or.jp/modules/en/index.php?content_id=44. Accessed Apr 30, 2015.
Pharmaceutical and Food Safety Bureau. English translation of Attachment 1 of Division-Notification 0229 No. 10, February 29, 2012. Guideline for Bioequivalence Studies of Generic Products. http://www.nihs.go.jp/drug/be-guide(e)/Generic/GL-E_120229_BE.pdf. Accessed Mar 13, 2015.
Sohda T, Ikeda H, Meguro K. Studies on antidiabetic agents. XII. Synthesis and activity of the metabolites of (±)-5(-)[p(-)[2-(5-ethyl-2-pyridyl)ethoxy]benzyl]-2,4- thiazolidinedione (pioglitazone). Chem Pharm Bull (Tokyo). 1995;43:2168–72.
Macha S, Dieterich S, Mattheus M, Seman LJ, Broedl UC, Woerle HJ. Pharmacokinetics of empagliflozin, a sodium glucose cotransporter-2 (SGLT2) inhibitor, and metformin following co-administration in healthy volunteers. Int J Clin Pharmacol Ther. 2013;51:132–40. doi:10.5414/cp201794.
Scheen AJ. Drug-drug interactions with sodium-glucose cotransporters type 2 (SGLT2) inhibitors, new oral glucose-lowering agents for the management of type 2 diabetes mellitus. Clin Pharmacokinet. 2014;53:295–304. doi:10.1007/s40262-013-0128-8.
Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53:213–25. doi:10.1007/s40262-013-0126-x.
Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012;7:e30555. doi:10.1371/journal.pone.0030555.
Uejima E, Takahashi K, Morisaki T, Ohno M, Nishida Y, Moriya M, et al. Microsomal enzyme induction and clinical aggravation of porphyria: the evaluation of human urinary 6beta-hydroxycortisol/cortisol ratio as the index of hepatic CYP3A4 activity. J Clin Pharmacol. 2002;42:1374–9.
Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complicat. 2013;27:473–8. doi:10.1016/j.jdiacomp.2013.05.004.
Nicolle LE, Capuano G, Ways K, Usiskin K. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin. 2012;28:1167–71. doi:10.1185/03007995.2012.689956.
Seino Y, Inagaki N, Haneda M, Kaku K, Sasaki T, Fukatsu A, et al. Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2015. doi:10.1111/jdi.12316.
Acknowledgments
Sponsorship, article processing charges, and the open access charge for this study were funded by Taisho Pharmaceutical Co., Ltd. The authors wish to thank the subjects for participating in the studies and the investigators and clinic staff for performing the studies. The authors also thank Nicholas D. Smith, PhD (Edanz Group Ltd.) for editorial support, which was funded by Taisho Pharmaceutical Co., Ltd. Soichi Sakai is the guarantor for this article, and takes responsibility for the integrity of the work as a whole. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Conflict of interest
T Sasaki has received research funds from Canon Inc. and Taisho Toyama Pharmaceutical Co., Ltd., and consulting fees or lecture fees from Taisho, Taisho Toyama, Sanofi, Kowa, Mitsubishi-Tanabe, Novo Nordisk Pharma, MSD and the LIGHT Study group (LIGHT Study; UMIN ID 000015112). Y Seino has received consulting fees or lecture fees from Sanofi, Novo Nordisk, Eli Lilly, GlaxoSmithKline, Astellas, Takeda, Boehringer Ingelheim, Johnson & Johnson, Becton–Dickinson, AstraZeneca, Taisho Toyama and Taisho. A Fukatsu has received consulting fees or lecture fees from Taisho Toyama and Taisho. M Ubukata is an employee of Taisho. S Sakai is an employee of Taisho. Y Samukawa is an employee of Taisho.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2000 and 2008. The relevant study protocol was approved by the Institutional Review Board at the participating institution. All the patients provided informed consent to participate in this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Results of these studies were reported as a poster Sasaki T, Seino Y, Samukawa Y, et al. Drug–drug Interactions of Luseogliflozin (TS-071) with Other Oral Hypoglycemic Agents in Healthy Japanese Subjects. 9th IDF-WPR Congress & 4th AASD Scientific Meeting, Kyoto, Japan, November 24–27, 2012. Poster Session PCS-33-8.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sasaki, T., Seino, Y., Fukatsu, A. et al. Absence of Drug–Drug Interactions Between Luseogliflozin, a Sodium–Glucose Co-transporter-2 Inhibitor, and Various Oral Antidiabetic Drugs in Healthy Japanese Males. Adv Ther 32, 404–417 (2015). https://doi.org/10.1007/s12325-015-0209-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0209-1