Abstract
Introduction
The 21-gene breast cancer assay (Oncotype DX®; Genomic Health, Inc.) is a validated diagnostic test that predicts the likelihood of adjuvant chemotherapy benefit and 10-year risk of distant recurrence in patients with hormone-receptor-positive, human epidermal growth receptor 2-negative, early-stage breast cancer. The aim of this analysis was to evaluate the cost-effectiveness of using the assay to inform adjuvant chemotherapy decisions in Mexico.
Methods
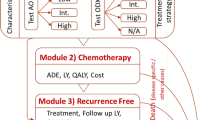
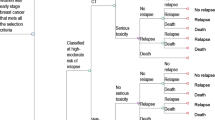
A Markov model was developed to make long-term projections of distant recurrence, survival, and direct costs in scenarios using conventional diagnostic procedures or the 21-gene assay to inform adjuvant chemotherapy recommendations. Transition probabilities and risk adjustment were taken from published landmark trials. Costs [2011 Mexican Pesos (MXN)] were estimated from an Instituto Mexicano del Seguro Social perspective. Costs and clinical benefits were discounted at 5% annually.
Results
Following assay testing, approximately 66% of patients previously receiving chemotherapy were recommended to receive hormone therapy only after consideration of assay results. Furthermore, approximately 10% of those previously allocated hormone therapy alone had their recommendation changed to add chemotherapy. This optimized therapy allocation led to improved mean life expectancy by 0.068 years per patient and increased direct costs by MXN 1707 [2011 United States Dollars (USD) 129] per patient versus usual care. This is equated to an incremental cost-effectiveness ratio (ICER) of MXN 25,244 (USD 1914) per life-year gained.
Conclusion
In early-stage breast cancer patients in Mexico, guiding decision making on adjuvant therapy using the 21-gene assay was projected to improve life expectancy in comparison with the current standard of care, with an ICER of MXN 25,244 (USD 1914) per life-year gained, which is within the range generally considered cost-effective.


Similar content being viewed by others
References
Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358(3):213–6.
Rodríguez-Cuevas S, Macías C, Labastida S. Cáncer de mama en México: ¿enfermedad de mujeres jóvenes? Ginecol Obstet Mex. 2000;68(5):185–90.
Knaul FM, Nigenda G, Lozano R, Arreola-Ornelas H, Langer A, Frenk J. Breast cancer in Mexico: a pressing priority. Reprod Health Matters. 2008;16(32):113–23.
Knaul FM, Arreola-Ornelas H, Velázquez E, Dorantes J, Méndez O, Avila-Burgos L. The health care costs of breast cancer: the case of the Mexican Social Security Institute. Salud Publica Mex. 2009;51(Suppl 2):s286–95.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34.
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Hassett MJ, Hughes ME, Niland JC, et al. Chemotherapy use for hormone receptor–positive, lymph node–negative breast cancer. J Clin Oncol. 2008;26(34):5553–60.
Hornberger J, Chien R. Meta-analysis of the decision impact of the 21-gene breast cancer recurrence score in clinical practice. In: San Antonio Breast Cancer Symposium 2010. Poster Presentation #P3-09-06.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–34.
Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in a randomized trial of chemotherapy for postmenopausal, node-positive, estrogen receptor-positive breast cancer. Lancet Oncol. 2010;11(1):55–65.
Aebi S, Davidson T, Gruber G, Castiglione M, ESMO Guidelines Working Group. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v9–14.
Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
NCCN Clinical Practice Guidelines in Oncology™ Breast Cancer, (Version 1.2011). Available at: http://www.nccn.org. Accessed Mar 4, 2013.
Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol. 2009;20(8):1319–29.
Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–47.
Azim HA Jr, Michiels S, Zagouri F, et al. Utility of prognostic genomic tests in breast cancer practice: the IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013;24(3):647–54.
Rouzier R, Pronzato P, Chéreau E, Carlson J, Hunt B, Valentine WJ. Multigene assays and molecular markers in breast cancer: systematic review of health economic analyses. Breast Cancer Res Treat. 2013;139(3):621–37.
Holt SDH, Bennett H, Bertelli G, Valentine WJ, Phillips CJ. Cost-effectiveness of the Oncotype DX® breast cancer assay in clinical practice in the UK. In: Poster presented at the 34th Annual San Antonio Breast Cancer Symposium, 2011.
International Society for Health Economics and Outcomes Research. Pharmacoeconomic guidelines around the world. Available at http://www.ispor.org/PEguidelines/countrydet.asp?c=37&t=1. Accessed Mar 12, 2013.
Secretaría de Salud. http://sinais.salud.gob.mx. Accessed Nov 13, 2012.
Thomas RJ, Williams M, Marshall C, Glen J, Callam M. The total hospital and community UK costs of managing patients with relapsed breast cancer. Br J Cancer. 2009;100(4):598–600.
Instituto Nacional de Cancerología. http://incan-mexico.org/. Accessed Nov 13, 2012.
Gómez-Rico JA, Altagracia-Martínez M, Kravzov-Jinich J, Cárdenas-Elizalde R, Rubio-Poo C. The costs of breast cancer in a Mexican public health institution. Risk Manag Healthcare Pol. 2008;1:15–21.
Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 2001;30:135–42.
Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health. 2012;15(6):835–42.
Remák E, Brazil L. Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer. 2004;91(1):77–83.
Bargallo JER, Lara F, Shaw Dulin RJ, et al. A study of the impact of the 21-gene breast cancer assay on the use of adjuvant chemotherapy in women with breast cancer in a Mexican public hospital. In: Poster presented at European Society for Medical Oncology Congress, 2012.
Conner-Spady BL, Cumming C, Nabholtz JM, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant. 2005;36(3):251–9.
Canadian Breast Cancer Network. Breast Cancer: Economic Impact and Labour Force Re-Entry. 2010. Available at: http://www.cbcn.ca/index.php?pageaction=content.page&id=2912&lang=en. Accessed Nov 17, 2011.
Peugniez C, Fantoni S, Leroyer A, Skrzypczak J, Duprey M, Bonneterre J. Return to work after treatment for breast cancer: single center experience in a cohort of 273 patients. Bull Cancer. 2011;98(7):E69–79.
López-Carrillo L, Torres-Sánchez L, López-Cervantes M, et al. Identificación de lesions mamarias en México. Salud Pública de México. 2001;43(3):199–202.
O’Leary B, Yoshizawa C, Foteff C, Chao C. Cost-effectiveness of the Oncotype DX assay in Australia: an exploratory analysis. In: Presented at ISPOR 4th Asia-Pacific Conference, Phuket. 2010.
Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11(5):313–24.
Madaras B, Rózsa P, Gerencsér Z, et al. The Impact of chemotherapeutic regimens on the cost-utility analysis of Oncotype DX assay. In: Presented at EBCC 8, Vienna. 2012.
Klang SH, Hammerman A, Liebermann N, Efrat N, Doberne J, Hornberger J. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health. 2010;13(4):381–7.
de Lima Lopes G, Chien R, Hornberger JC. Cost-benefit analysis of a 21-gene recurrence score for early-stage breast cancer in Singapore. In: Presented at 12th St. Gallen International Breast Cancer Conference, St Gallen. 2011.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Genomic Health, Inc. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interest
Juliette Plun-Favreau is an employee of Genomic Health International. William Valentine is an employee of Ossian Health Economics and Communications. Barnaby Hunt is an employee of Ossian Health Economics and Communications. Ossian received funding from Genomic Health International to support the present study. Juan Enrique Bargalló-Rocha, Fernando Lara-Medina, Victor Pérez-Sánchez, Rafael Vázquez-Romo, Cynthia Villarreal-Garza, Hector Martínez-Said, Robin J Shaw-Dulin, and Alejandro Mohar-Betancourt have no conflicts of interest to declare.
Compliance with ethics guidelines
The analysis in this article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bargalló-Rocha, J.E., Lara-Medina, F., Pérez-Sánchez, V. et al. Cost-Effectiveness of the 21-Gene Breast Cancer Assay in Mexico. Adv Ther 32, 239–253 (2015). https://doi.org/10.1007/s12325-015-0190-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0190-8