Abstract
In experimental studies, mesenchymal stem cell (MSC) transplantation in acute myocardial infarction (AMI) models has been associated with enhanced neovascularization and myogenesis. Clinical data however, are scarce. Therefore, the present study evaluates the safety and feasibility of intramyocardial MSC injection in nine patients, shortly after AMI during short-term and 5-year follow-up. Periprocedural safety analysis demonstrated one transient ischemic attack. No other adverse events related to MSC treatment were observed during 5-year follow-up. Clinical events were compared to a nonrandomized control group comprising 45 matched controls. A 5-year event-free survival after MSC-treatment was comparable to controls (89 vs. 91 %, P = 0.87). Echocardiographic imaging for evaluation of left ventricular function demonstrated improvements up to 5 years after MSC treatment. These findings were not significantly different when compared to controls. The present safety and feasibility study suggest that intramyocardial injection of MSC in patients shortly after AMI is feasible and safe up to 5-year follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the use of state-of-the-art invasive and pharmacological treatment strategies, myocardial infarction (MI) remains a major cause of morbidity and mortality worldwide. Over the last decade, several nonrandomized and randomized studies have demonstrated that transplantation of bone marrow-derived cells shortly after acute MI may result in improved cardiac function and reduced infarct size [1]. Therefore, cardiac stem and progenitor cell transplantation is currently under investigation as a potential therapeutic option for acute MI patients.
Mesenchymal stem cells (MSCs), a subpopulation of bone marrow cells, were found to be able to differentiate into various cell types including vascular cells and, under specific in vitro conditions, functional cardiomyocytes [2, 3]. In preclinical models of acute MI, MSC transplantation is reported to promote neovascularization and myogenesis, resulting in improved myocardial function [2, 4]. Early clinical studies documented that intracoronary and intravenous infusion of MSCs shortly after acute MI is safe and feasible [5, 6]. Moreover, a beneficial effect on global and regional left ventricular (LV) function was presumed. Recently, Williams et al. suggested that catheter-based intramyocardial MSC injection in patients with chronic ischemic heart disease is safe and results in a reduced perfusion defect size and an improved contractility after 12 months [7].
Until now, no clinical study assessed the safety and feasibility of catheter-based intramyocardial MSC injection shortly after acute MI. Conceptually, intramyocardial injection may be more suitable for the delivery of large cell types such as MSCs without the potential risk of micro-embolism formation as reported after intracoronary delivery of MSCs in dogs [8] and umbilical cord-derived stem cells in pigs [9]. Furthermore, intramyocardial cell delivery has been associated with higher retention rate of injected cells as compared to intracoronary and intravenous infusion [10] culminating in potential greater beneficial effect on the myocardium.
The aim of the present study was to determine the short- and long-term safety and feasibility of intramyocardial autologous bone marrow-derived, ex vivo expanded, MSC injection in patients shortly (<4 weeks) after acute ST-segment elevation MI. Furthermore, the effect on cardiac function and myocardial perfusion was evaluated at short- and long-term follow-up and compared to a historical control group.
Methods
Patient Population
Patients with a first acute ST-segment elevation MI treated by primary percutaneous coronary intervention (PCI) of the infarct-related coronary artery <12 h after onset of chest pain were eligible for inclusion in the current study if the maximal CK level was >1,600 U/L. Exclusion criteria were prior MI and/or coronary artery bypass grafting, a history of malignancy, the presence of an unstable medical situation, a remaining significant coronary lesion for which an additional revascularization procedure was warranted, significant co-morbidity (e.g., renal dysfunction [glomerular filtration rate, <30 ml/min/1.73 m2], significant valvular heart disease, active infection), concurrent participation in another study, or unwillingness to participate. The institutional ethical committee approved the study protocol and written informed consent was obtained from all patients. The study was registered at the Dutch trial registry (www.trialregister.nl, no. NTR1553).
Study Protocol
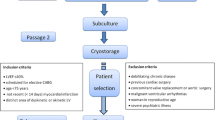
The study protocol of this safety and feasibility study is displayed in Fig. 1. The baseline evaluation consisted of two-dimensional (2D) echocardiography (<24–48 h of admission for acute MI) and stress–rest single-photon emission computed tomography (SPECT; approximately 2 weeks after acute MI).
Bone marrow was aspirated from the iliac crest under local anesthesia within 7 days after the infarction. Following mononuclear cell isolation, the MSCs were expanded ex vivo for 14–24 days, after which the intramyocardial injection procedure was performed. For this purpose, a three-dimensional electromechanical map of the LV was generated with the use of the NOGA system (Biologics Delivery Systems Group, Cordis; Bridgewater, NJ, USA). After completion of the electromechanical map of the LV, the mapping catheter was replaced by an injection catheter (MyoStar catheter, Biosense-Webstar) and MSC injections were targeted in the infarct border zone. Subsequently, 8–12 intramyocardial injections of approximately 0.2–0.5 mL each were performed with the NOGA system as previously described [11]. After the procedure, continuous heart rhythm monitoring was started for 2 days and laboratory markers of myocardial damage were collected. To assess potential postprocedural pericardial effusion, echocardiography was performed 1 day after the injection procedure.
After discharge, the clinical status was assessed at the outpatient clinic at 3, 6, and 12 months follow-up. To monitor the occurrence of ventricular arrhythmias 24-h Holter monitoring was performed at 3 and 6 months follow-up. LV function was determined with the use of 2D echocardiography at 3, 6, and 12 months and myocardial perfusion was assessed at 3 months. After the first year of follow-up, patients were evaluated at the outpatient clinic on a yearly basis to assess the occurrence of clinical events (death, MI, revascularization, and hospitalization for heart failure). At 4–5 year follow-up, an additional evaluation was performed containing a 24-h Holter recording and 2D echocardiography. From patients who died during follow-up, hospital records were reviewed and the cause of death was ascertained.
Mesenchymal Stem Cell Isolation and Expansion
Ex vivo expansion of mononuclear cells was performed according to a protocol devised by the European Group for Blood and Bone Marrow Transplantation developmental committee, as previously described [12]. In summary, after bone marrow aspiration, mononuclear cells were isolated by Ficoll density gradient centrifugation (density, 1.077 g/cm3) and were washed and resuspended in Dulbecco’s modified Eagle’s low-glucose medium (Invitrogen, Paisley, UK) supplemented with penicillin and streptomycin (Lonza, Verviers, Belgium) and 10 % fetal bovine serum (HyClone, Logan, UT, USA), without any additional growth factors. Mononuclear cells were plated at a density of 160,000 cells/cm2 and cultures were maintained at 37 °C in a humidified atmosphere containing 5 % CO2 in 175 cm2 flasks (Greiner Bio-One, Frickenhausen, Germany). The cells were detached and replated when the cultures reached near confluence (>80 %). MSCs were passaged up to a maximum of two times. At the day of cell injection, the cells were harvested and were suspended in a 5 mL (or 2.5 mL if final cell count was <10 × 106 cells) isotonic buffer with human serum albumin or human autologous serum and packaged in 2.5 mL syringe for intramyocardial injection. The expanded cell population was tested for sterility as well as the presence of mycoplasma genus by PCR. Culture expanded MSCs were characterized by morphological examination and immunophenotying using a panel of membrane markers (HLA-I, HLA-DR, CD 31, CD 34, CD 45, CD 73, CD 80, CD 90, and CD 105).
SPECT Imaging
For SPECT imaging, a previously described 2-day outpatient stress–rest protocol was used [13]. In brief, during the stress protocol, adenosine (0.14 mg/kg/min) was infused for 6 min and 500 MBq Tc-99m tetrofosmin was injected intravenously after 3.5 min of adenosine. A second injection of 500 MBq technetium-99m tetrofosmin was performed to obtain resting images. Myocardial perfusion was analyzed by reconstructing a standard short- and long-axis projection, perpendicular to the heart axis, which was adjusted for peak myocardial activity (100 %).
Myocardial perfusion was analyzed using Quantitative Gated SPECT software (QGS software, Cedars-Sinai Medical Center, Los Angeles, CA, USA). The myocardium was divided into 17 segments and segmental tracer activity was categorized on a four-point scale: 1 = tracer activity, >75 %; 2 = tracer activity, 50–75 %; 3 = tracer activity, 25–49 %; and 4 = tracer activity, <25 %. Significant fill-in (>10 %) of perfusion defects observed on the images at rest was classified as ischemic myocardium. The summed stress score and summed rest score were calculated by summation of the patients’ segmental scores, respectively, at stress and rest. All measurements were performed by two experienced observers (J.v.R. and S.F.R),
Echocardiography
Patients were imaged in the left lateral decubitus position using a commercially available system (Vivid 7 and E9, General Electric-Vingmed, Horten, Norway). Data acquisition was performed with a 3.5-MHz transducer at a depth of 16 cm in the standard parasternal and aprical views. During breath hold, M-mode and 2D images were obtained and saved in cine-loop format. Data analysis was performed offline (EchoPAC version 111.0.0; General Electric-Vingmed,). End-diastolic volumes, end-systolic volumes, and LV ejection fractions were derived from the conventional 2D apical two- and four-chamber images, using the biplane Simpson’s rule. A 16-segment model of the LV was used to assess regional wall motion and wall motion score index by a four-point scale calculation (1 = normokinesia, 2 = hypokinesia, 3 = akinesia, 4 = dyskinesia). Previous work has reported on the inter- and intravariabilities in our laboratory [14].
Historical Control Group
In order to provide more insight in the safety of intramyocardial MSC injection after acute MI, a historical control group was included retrospectively consisting of patients with a first ST-segment elevation acute MI, successfully treated with primary PCI, during the study period who matched the inclusion and exclusion criteria of the study, but who could not be included because of logistical reasons. For each patient included for MSC treatment, five controls were included that were matched for age-, gender-, and infarct-related coronary artery.
Control patients were monitored at the regular post-infarction outpatient clinic at 3, 6, and 12 months follow-up. The standardized follow-up of these patients included 2D echocardiography at 3, 6, and 12 months follow-up (similar as in MSC-treated patients). After the first year of follow-up, patients were monitored by either outpatient clinic visits and/or telephone inquiry [15].
Statistical Analysis
Data are reported as mean ± SD. Continuous variables were compared using a Wilcoxon signed-rank test or Mann–Whitney test. Categorical data were compared using a Fisher’s exact test. A Friedman test was applied to analyze echocardiographic results of MSC-treated patients at >2 time points. To compare the change in echocardiographic results during 12 months follow-up between MSC-treated patients and controls, a two-way repeated measures analysis of variance was applied. For comparison of echocardiographic results, a complete case analysis was used. Clinical outcome was assessed by Kaplan–Meier analysis, for which only the first event of each patient was included into the analysis. Differences between event-free survival curves were tested with the log-rank statistic. A P value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS software version 17.0 (SPSS, Chicago, IL, USA).
Results
Between 2006 and 2008, a total of nine patients (age 56 ± 8 years) were treated by intramyocardial autologous MSC injection. The baseline data of these nine patients and the 45 historical controls (age 60 ± 11 years) are summarized Table 1. As indicated, echocardiographically assessed LV ejection fraction was 48 ± 3 % in MSC-treated patients and 44 ± 10 % in the controls (P = 0.33).
Mesenchymal Stem Cell Expansion
Bone marrow aspiration resulted in 123 ± 17 ml of bone marrow. After 18.6 ± 3.5 days of culturing, the expansion procedure yielded 46 ± 3 × 106 MSCs with a viability of 93 ± 5 %. Immunophenotypical characterization of the MSC products showed expression of HLA-I, CD73, CD90, and CD105 surface molecules and absence of expression for CD34, CD45, CD31, CD80, and HLA DR (data not shown) in compliance with the definition of MSC by the International Society for Cellular Therapy [16]. Injected per patient was 31 ± 2 × 106 MSCs (Table 2).
Periprocedural Data
Intramyocardial MSC injection was performed 21 ± 3 days after infarction. Mean procedural time for LV mapping and cell injection was 71 ± 23 min. Patients received a mean of 11 ± 1 intramyocardial injections.
One patient experienced a transient ischemic attack 2 h after the injection procedure and developed a recurrence of atrial fibrillation during this transient ischemic attack that was successfully treated by electrical cardioversion. During hospitalization, repetitive laboratory measurements revealed no postprocedural MI or systemic inflammation. In addition, sustained ventricular tachycardias were not observed and 2D echocardiography did not reveal pericardial effusion. All patients were discharged 48 h after the injection procedure.
Safety Data of MSC Injection
During the study period, one patient died at 9 months follow-up due to postoperative infectious complications after a surgical LV reconstruction, mitral, and tricuspid valve annuloplasty which was performed because of progressive heart failure and LV dysfunction. This serious adverse event was considered not to be related to the MSC therapy. In summary, this diabetic patient had a large anterior MI with an 11-h delay to PCI. Prior to the injection procedure, echocardiography had shown a developing apical aneurysm for which the patient was started on oral anticoagulant treatment. During follow-up after cell injection, the patient experienced increasing heart failure related complaints, up to New York Heart Association class III/IV, with an LV ejection fraction that gradually decreased from 43 % at baseline to 17 % at the time of surgery. Microscopic evaluation of myocardial samples obtained at autopsy did not reveal any calcification or osteoid depositions. Furthermore, the von Kossa staining, used to quantify mineralization in tissue, could not demonstrate any calcium depositions in the myocardium.
Throughout the study period, none of the patients experienced a recurrent MI. In two patients, a PCI was performed at 4 months respectively 4 years of follow-up because of progressive stable angina pectoris both due to a new stenosis of a nonculprit coronary artery located outside the MSC-injected area of the myocardium. For this reason, these events were also considered not to be related to the MSC injection.
Repeated Holter monitoring revealed no ventricular arrhythmias during the 4–5 year follow-up period. Of interest, at long-term follow-up, none of the patients had symptoms or signs of heart failure.
Long-Term Follow-up in MSC-Treated Patients and Historical Controls
At 4–5 year follow-up, the survival was similar between MSC-treated patients and historical controls (89 vs. 89 %, P = 0.97). Furthermore, the percentage of major coronary events (MI or revascularization procedures) was comparable in both groups (22 vs. 18 %, P = 0.88). The Kaplan–Meier curves of event-free survival for death, MI, revascularization, and admission for heart failure are shown in Fig. 2.
Myocardial Perfusion Assessed by SPECT
Paired SPECT studies were available for all nine MSC-treated patients at 3 months follow-up. As shown in Fig. 3, the summed stress score improved from 33 ± 7 at baseline to 30 ± 7 at 3 months (P < 0.01). Similarly, the summed rest score improved from 31 ± 8 to 29 ± 8 at 3 months (P = 0.04). The number of ischemic segments significantly decreased from 1.6 ± 1.9 to 0.7 ± 1.4 at 3 months (P = 0.02).
Echocardiography
Echocardiography at baseline and 3, 6, and 12 months follow-up was available in eight MSC-treated patients and in 38 control patients. Table 3 displays the results of LV function assessment during the follow-up period. In MSC-treated patients, there was an improvement in LV ejection fraction from 48 ± 2 % at baseline to 52 ± 2 % at 3 months and 57 ± 3 % at 12 months (P < 0.01). The magnitude of improvement is similar to the improvement observed in historical controls (Fig. 4; P = 0.28).
Echocardiography at long-term follow-up (4.3 ± 1.3 years) was available in eight MSC-treated patients and revealed that the improvement in LV ejection fraction was sustained (55 ± 7 %, P = 0.41 vs. 12 months). Table 2 displays the results of LV function assessment during follow-up of individual MSC-treated patients.
Discussion
The present study is the first clinical trial to evaluate the feasibility and long-term (up to 5 years) safety of intramyocardial injection of bone marrow-derived, ex vivo-expanded MSCs shortly after acute MI. The main finding from the current study is that MSC treatment is feasible and safe at short term and up to 5 years of follow-up. Furthermore, the 5-year event-free survival was not different from the control group. Global LV function assessed by echocardiography showed continuous improvements in LV systolic function after MSC injection during the first 12 months that were comparable to the control group and which were maintained up to 5 years post-MI.
Bone marrow-derived MSCs are a promising cell type for cell-based therapy. In preclinical studies, MSC transplantation resulted in improved LV function, increased capillary density and reduced infarct size in models of acute MI [2, 17, 18]. Since in vivo differentiation of naive MSCs into new cardiomyocytes remains controversial [19], these beneficial effects are most likely related to stimulation of angiogenesis by differentiation of MSCs into endothelial and smooth muscle cells [2, 4] and by paracrine mechanisms, including secretion of a variety of growth factors and cytokines that contribute to cardiac repair. Interestingly, MSCs also have the ability to home to sites of acute tissue injury [20], the potential to actively suppress the immune system and reduce inflammation [21, 22], and thereby possibly inducing repair and contributing to functional improvement after acute MI.
Although bone marrow aspiration is well tolerated, culture times from 2 to 5 weeks prohibit the acute therapeutic use of autologous bone marrow-derived MSCs. Precultured and cryopreserved allogeneic MSCs might be considered as alternative in this respect. Allogeneic bone marrow MSC, however, might be liable for immune rejection [23, 24], while both autologous and allogeneic bone marrow MSCs require dedicated staff and certified culturing facilities which can be logistically challenging. Although these bone marrow MSC associated hurdles might be addressed using bioreactors [25], autologous adipose tissue-derived cell (ADSCs) therapy is presently investigated as a promising alternative. ADSCs can be harvested in large amounts without a need for further culturing and have been demonstrated to express similar surface markers and a comparable self-renewal and differentiation capacity as bone marrow-derived MSCs [26, 27]. Preclinical studies demonstrated beneficial effects on LV function and myocardial perfusion after transplantation of ADSCs in acute MI models [28, 29] and the first-in-men safety and feasibility study is currently underway [26].
Clinical experience with MSCs for cardiac cell therapy after acute MI is limited, comprising a number of small-sized clinical studies in which different administration routes have been used [5, 6, 30]. For example, in a clinical trial performed by Chen et al. which randomized patients with acute MI to intracoronary infusion of autologous MSCs or placebo, MSC treatment was associated with beneficial effects on LV function and myocardial perfusion [5]. In another randomized trial by Hare et al., intravenous infusion of allogeneic MSCs in patients with acute MI resulted in improved LV systolic function after cell treatment in a subgroup of anterior MI patients as compared to placebo [6]. Importantly, the abovementioned studies did not document serious MSC-related adverse events but the follow-up was limited to 6 months.
The current study is the first study using the intramyocardial injection technique for MSC delivery in patients with acute MI. Catheter-based transendocardial injection of bone marrow-derived cells has been performed in several studies in patients with chronic MI or refractory angina [7, 11, 31, 32], which demonstrated that this technique is feasible and safe, also after long-term follow-up. An advantage of this technique is that viability of potential injection sites can be assessed, allowing accurate targeting of cell injections into the infarct border zone or ischemic myocardium [33, 34]. Furthermore, intramyocardial injection is suggested to result in higher rates of cell retention as compared to intracoronary or intravenous infusion [35], possibly culminating in a larger functional improvement [36]. Finally, intramyocardial injection avoids the potential risk of coronary obstruction and microinfarction, which is considered to be a risk of intracoronary MSC infusion [8]. Trans-endocardial injection procedures on the other hand might theoretically be less safe in recently infarcted myocardium. The unremarkable short and long follow-up in our patients however indicates the safety of the transendocardial injection procedure. Specifically, no malignant arrhythmias occurred during or after the procedure and no pericardial effusion was observed on echocardiography 1 day after the injection procedure. In agreement, no events were observed in a recent study on 20 patients during 12 months follow-up of trans-endocardial bone marrow-derived mononuclear cell injection in patient shortly after acute MI [37].
In previous trials, safety of MSC administration has been reported up to 12 months follow-up [7]. The current study is the first to report safety results up to 5 years after MSC administration. Importantly, the event-free survival was not different between MSC-treated patients and the control group and no adverse events related to MSC treatment were observed. During follow-up, 24-h Holter monitoring revealed no ventricular arrhythmias and echocardiography did not detect any myocardial tumor formation. However, one patient gradually developed therapy resistant heart failure and died 9 months after MSC injection as a result of a septic shock during a prolonged ICU stay following extensive heart failure surgery. Although the cause of death is not MSC-related, an MSC-related progression of the heart failure cannot be ruled out. Histological examination of the myocardium of this patient revealed no evidence for calcification or osteoid depositions in the myocardium. Furthermore, this diabetic patient had a large anterior MI with an 11-h delay to PCI, which are established risk factors for the development of extensive LV remodeling [38].
Evaluation of LV function by echocardiography did not demonstrate significant additional improvements in LV function in MSC-treated patients as compared to medically treated control patients in the first 12 months follow-up. Obviously, given the small number of MSC-treated patients, the study is not powered to detect a treatment effect on LV function. Also, the use of serial magnetic imaging rather than echocardiography is more suitable to detect small improvements in LV volumes [39]. Nonetheless, from a safety point of view, the results from echocardiography during the first 12 months and at long-term follow-up demonstrate that MSC injection is not associated with detrimental effects on LV systolic function. SPECT imaging revealed an improvement in myocardial perfusion during rest and exercise 3 months after MSC injection. However, since SPECT imaging was not repeated in the control group, the effect of MSC treatment on myocardial perfusion can therefore not be evaluated.
Study Limitations
The current study has several limitations. Most important, small relative risks of MSC therapy will not be observed in the limited number of with MSC-treated patients. Moreover, the comparison with a matched but nonrandomized control group is not ideal and liable for confounding. Besides this general limitation of the control group, functional data for LV functionality for both controls and treated patients showed to be similarly improved at 12 months follow-up.
Conclusion
In summary, the results of the current safety and feasibility study demonstrates that intramyocardial injection of autologous bone marrow-derived, ex vivo expanded, MSCs in patients shortly after acute MI is feasible and safe at short- and long-term (up to 5 years) follow-up. Based on the safety results of the current study and the promising preclinical findings, we feel that further randomized, placebo-controlled trials are justified to assess the efficacy of intramyocardial MSC injection in acute MI patients.
Abbreviations
- MI:
-
Myocardial infarction
- MSCs:
-
Mesenchymal stem cells
- LV:
-
Left ventricular
- PCI:
-
Percutaneous coronary intervention
- SPECT:
-
Single-photon emission computed tomography
References
Jeevanantham, V., Butler, M., Saad, A., Abdel-Latif, A., Zuba-Surma, E. K., & Dawn, B. (2012). Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation, 126, 551–568.
Silva, G. V., Litovsky, S., Assad, J. A., Sousa, A. L., Martin, B. J., Vela, D., Coulter, S. C., Lin, J., Ober, J., Vaughn, W. K., Branco, R. V., Oliveira, E. M., He, R., Geng, Y. J., Willerson, J. T., & Perin, E. C. (2005). Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation, 111, 150–156.
Makino, S., Fukuda, K., Miyoshi, S., Konishi, F., Kodama, H., Pan, J., Sano, M., Takahashi, T., Hori, S., Abe, H., Hata, J., Umezawa, A., & Ogawa, S. (1999). Cardiomyocytes can be generated from marrow stromal cells in vitro. The Journal of Clinical Investigation, 103, 697–705.
Dai, W., Hale, S. L., Martin, B. J., Kuang, J. Q., Dow, J. S., Wold, L. E., & Kloner, R. A. (2005). Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation, 112, 214–223.
Chen, S. L., Fang, W. W., Ye, F., Liu, Y. H., Qian, J., Shan, S. J., Zhang, J. J., Chunhua, R. Z., Liao, L. M., Lin, S., & Sun, J. P. (2004). Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. The American Journal of Cardiology, 94, 92–95.
Hare, J. M., Traverse, J. H., Henry, T. D., Dib, N., Strumpf, R. K., Schulman, S. P., Gerstenblith, G., DeMaria, A. N., Denktas, A. E., Gammon, R. S., Hermiller, J. B., Jr., Reisman, M. A., Schaer, G. L., & Sherman, W. (2009). A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology, 54, 2277–2286.
Williams, A. R., Trachtenberg, B., Velazquez, D. L., McNiece, I., Altman, P., Rouy, D., Mendizabal, A. M., Pattany, P. M., Lopera, G. A., Fishman, J., Zambrano, J. P., Heldman, A. W., & Hare, J. M. (2011). Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circulation Research, 108, 792–796.
Vulliet, P. R., Greeley, M., Halloran, S. M., MacDonald, K. A., & Kittleson, M. D. (2004). Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet, 363, 783–784.
Moelker, A. D., Baks, T., Wever, K. M., Spitskovsky, D., Wielopolski, P. A., van Beusekom, H. M., van Geuns, R. J., Wnendt, S., Duncker, D. J., & van der Giessen, W. J. (2007). Intracoronary delivery of umbilical cord blood derived unrestricted somatic stem cells is not suitable to improve LV function after myocardial infarction in swine. Journal of Molecular and Cellular Cardiology, 42, 735–745.
Hou, D., Youssef, E. A., Brinton, T. J., Zhang, P., Rogers, P., Price, E. T., Yeung, A. C., Johnstone, B. H., Yock, P. G., & March, K. L. (2005). Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation, 112, I150–I156.
van Ramshorst, J., Bax, J. J., Beeres, S. L., Dibbets-Schneider, P., Roes, S. D., Stokkel, M. P., de Roos, A., Fibbe, W. E., Zwaginga, J. J., Boersma, E., Schalij, M. J., & Atsma, D. E. (2009). Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA : The Journal of the American Medical Association, 301, 1997–2004.
Le Blanc, K., Frassoni, F., Ball, L., Locatelli, F., Roelofs, H., Lewis, I., Lanino, E., Sundberg, B., Bernardo, M. E., Remberger, M., Dini, G., Egeler, R. M., Bacigalupo, A., Fibbe, W., & Ringden, O. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet, 371, 1579–1586.
Beeres, S. L., Bax, J. J., Dibbets, P., Stokkel, M. P., Zeppenfeld, K., Fibbe, W. E., van der Wall, E. E., Schalij, M. J., & Atsma, D. E. (2006). Effect of intramyocardial injection of autologous bone marrow-derived mononuclear cells on perfusion, function, and viability in patients with drug-refractory chronic ischemia. Journal of Nuclear Medicine, 47, 574–580.
Mollema, S. A., Liem, S. S., Suffoletto, M. S., Bleeker, G. B., van der Hoeven, B. L., van de Veire, N. R., Boersma, E., Holman, E. R., van der Wall, E. E., Schalij, M. J., Gorcsan, J., III, & Bax, J. J. (2007). Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. Journal of the American College of Cardiology, 50, 1532–1540.
Liem, S. S., van der Hoeven, B. L., Oemrawsingh, P. V., Bax, J. J., van der Bom, J. G., Bosch, J., Viergever, E. P., van Rees, C., Padmos, I., Sedney, M. I., van Exel, H. J., Verwey, H. F., Atsma, D. E., van der Velde, E. T., Jukema, J. W., van der Wall, E. E., & Schalij, M. J. (2007). MISSION!: optimization of acute and chronic care for patients with acute myocardial infarction. American Heart Journal, 153, 14–11.
Dominici, M., Le, B. K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., & Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Amado, L. C., Saliaris, A. P., Schuleri, K. H., St, J. M., Xie, J. S., Cattaneo, S., Durand, D. J., Fitton, T., Kuang, J. Q., Stewart, G., Lehrke, S., Baumgartner, W. W., Martin, B. J., Heldman, A. W., & Hare, J. M. (2005). Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America, 102, 11474–11479.
Beitnes, J. O., Oie, E., Shahdadfar, A., Karlsen, T., Muller, R. M., Aakhus, S., Reinholt, F. P., & Brinchmann, J. E. (2012). Intramyocardial injections of human mesenchymal stem cells following acute myocardial infarction modulate scar formation and improve left ventricular function. Cell Transplantation, 21, 1697–1709.
Toma, C., Pittenger, M. F., Cahill, K. S., Byrne, B. J., & Kessler, P. D. (2002). Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation, 105, 93–98.
Jain, M., Pfister, O., Hajjar, R. J., & Liao, R. (2005). Mesenchymal stem cells in the infarcted heart. Coronary Artery Disease, 16, 93–97.
Uccelli, A., Moretta, L., & Pistoia, V. (2006). Immunoregulatory function of mesenchymal stem cells. European Journal of Immunology, 36, 2566–2573.
Le Blanc, K., & Ringden, O. (2007). Immunomodulation by mesenchymal stem cells and clinical experience. Journal of Internal Medicine, 262, 509–525.
Huang, X. P., Sun, Z., Miyagi, Y., McDonald, K. H., Zhang, L., Weisel, R. D., & Li, R. K. (2010). Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation, 122, 2419–2429.
Nauta, A. J., Westerhuis, G., Kruisselbrink, A. B., Lurvink, E. G., Willemze, R., & Fibbe, W. E. (2006). Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood, 108, 2114–2120.
Chen, X., Xu, H., Wan, C., McCaigue, M., & Li, G. (2006). Bioreactor expansion of human adult bone marrow-derived mesenchymal stem cells. Stem Cells, 24, 2052–2059.
Meliga, E., Strem, B. M., Duckers, H. J., & Serruys, P. W. (2007). Adipose-derived cells. Cell Transplantation, 16, 963–970.
Planat-Benard, V., Silvestre, J. S., Cousin, B., Andre, M., Nibbelink, M., Tamarat, R., Clergue, M., Manneville, C., Saillan-Barreau, C., Duriez, M., Tedgui, A., Levy, B., Penicaud, L., & Casteilla, L. (2004). Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation, 109, 656–663.
Valina, C., Pinkernell, K., Song, Y. H., Bai, X., Sadat, S., Campeau, R. J., Le Jemtel, T. H., & Alt, E. (2007). Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European Heart Journal, 28, 2667–2677.
Schenke-Layland, K., Strem, B. M., Jordan, M. C., Deemedio, M. T., Hedrick, M. H., Roos, K. P., Fraser, J. K., & MacLellan, W. R. (2009). Adipose tissue-derived cells improve cardiac function following myocardial infarction. The Journal of Surgical Research, 153, 217–223.
Katritsis, D. G., Sotiropoulou, P. A., Karvouni, E., Karabinos, I., Korovesis, S., Perez, S. A., Voridis, E. M., & Papamichail, M. (2005). Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheterization and Cardiovascular Interventions, 65, 321–329.
Tse, H. F., Thambar, S., Kwong, Y. L., Rowlings, P., Bellamy, G., McCrohon, J., Thomas, P., Bastian, B., Chan, J. K., Lo, G., Ho, C. L., Chan, W. S., Kwong, R. Y., Parker, A., Hauser, T. H., Chan, J., Fong, D. Y., & Lau, C. P. (2007). Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial). European Heart Journal, 28, 2998–3005.
Losordo, D. W., Henry, T. D., Davidson, C., Sup, L. J., Costa, M. A., Bass, T., Mendelsohn, F., Fortuin, F. D., Pepine, C. J., Traverse, J. H., Amrani, D., Ewenstein, B. M., Riedel, N., Story, K., Barker, K., Povsic, T. J., Harrington, R. A., & Schatz, R. A. (2011). Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circulation Research, 109, 428–436.
Fuchs, S., Kornowski, R., Weisz, G., Satler, L. F., Smits, P. C., Okubagzi, P., Baffour, R., Aggarwal, A., Weissman, N. J., Cerqueira, M., Waksman, R., Serrruys, P., Battler, A., Moses, J. W., Leon, M. B., & Epstein, S. E. (2006). Safety and feasibility of transendocardial autologous bone marrow cell transplantation in patients with advanced heart disease. The American Journal of Cardiology, 97, 823–829.
Tse, H. F., Kwong, Y. L., Chan, J. K., Lo, G., Ho, C. L., & Lau, C. P. (2003). Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet, 361, 47–49.
Ang, K. L., Chin, D., Leyva, F., Foley, P., Kubal, C., Chalil, S., Srinivasan, L., Bernhardt, L., Stevens, S., Shenje, L. T., & Galinanes, M. (2008). Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nature Clinical Practice. Cardiovascular Medicine, 5, 663–670.
Brunskill, S. J., Hyde, C. J., Doree, C. J., Watt, S. M., & Martin-Rendon, E. (2009). Route of delivery and baseline left ventricular ejection fraction, key factors of bone-marrow-derived cell therapy for ischaemic heart disease. European Journal of Heart Failure, 11, 887–896.
Krause, K., Jaquet, K., Schneider, C., Haupt, S., Lioznov, M. V., Otte, K. M., & Kuck, K. H. (2009). Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart, 95, 1145–1152.
Antoni, M. L., Hoogslag, G. E., Boden, H., Liem, S. S., Boersma, E., Fox, K., Schalij, M. J., Bax, J. J., & Delgado, V. (2012). Cardiovascular mortality and heart failure risk score for patients after ST-segment elevation acute myocardial infarction treated with primary percutaneous coronary intervention (data from the Leiden MISSION! Infarct Registry). The American Journal of Cardiology, 109, 187–194.
Bellenger, N. G., Burgess, M. I., Ray, S. G., Lahiri, A., Coats, A. J., Cleland, J. G., & Pennell, D. J. (2000). Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? European Heart Journal, 21, 1387–1396.
Funding Sources
This work was supported by the Translational Regenerative Medicine (TeRM-Smart Mix SSM06004), the BioMedical Materials (SMARTCARE) program grants and the TAS research program 11.600.1016, which is financed by the Netherlands Organisation for Health Research and Development (ZonMW), the Netherlands.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Lorrie Kirshenbaum oversaw the review of this article
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rodrigo, S.F., van Ramshorst, J., Hoogslag, G.E. et al. Intramyocardial Injection of Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells in Acute Myocardial Infarction Patients is Feasible and Safe up to 5 Years of Follow-up. J. of Cardiovasc. Trans. Res. 6, 816–825 (2013). https://doi.org/10.1007/s12265-013-9507-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9507-7