Abstract
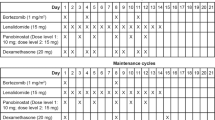
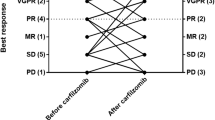
We report the final results from a multicenter, open-label phase I study of carfilzomib plus lenalidomide and dexamethasone in Japanese patients with heavily pretreated relapsed and/or refractory multiple myeloma (RRMM). Twenty-six RRMM patients were enrolled and received a median of 4.0 prior regimens; 12/26 patients (46.2%) completed the planned 18 administration cycles (mean number of cycles: 14.5 ± 4.9). The safety profile was consistent with that of previous carfilzomib studies. All patients experienced adverse events (AEs), but no new safety concerns were observed. The most common grade ≥ 3 AEs (incidence: ≥ 10%) were lymphocyte count decreased (46.2%), platelet count decreased (42.3%), and neutrophil count decreased (34.6%). The overall response rate was 88.5% (23/26; 90% confidence interval: 72.8–96.8). Complete response (CR) or better was achieved by 30.8% of patients compared with 3.8% in the interim analysis. The median time to CR or better response was 9.4 months. Median progression-free survival and duration of response were 19.5 months and 20.3 months, respectively. Median overall survival was not reached. Long-term administration of carfilzomib produced deep response and long-term disease control. The combination of carfilzomib plus lenalidomide and dexamethasone was well tolerated and showed promising clinical efficacy for heavily pretreated RRMM patients.
Clinical trial registration
This clinical trial was registered in the database clinicaltrials.jp (clinical trial registration number: Japic CTI 142677).




Similar content being viewed by others
References
Dingli D, Ailawadhi S, Bergsagel PL, Buadi FK, Dispenzieri A, Fonseca R, et al. Therapy for relapsed multiple myeloma: guidelines from the Mayo stratification for myeloma and risk-adapted therapy. Mayo Clin Proc. 2017;92:578–98.
Gonsalves WI, Milani P, Derudas D, Buadi FK. The next generation of novel therapies for the management of relapsed multiple myeloma. Future Oncol. 2017;13:63–75.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26:73–85.
Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–25.
Steiner RE, Manasanch EE. Carfilzomib boosted combination therapy for relapsed multiple myeloma. Onco Targets Ther. 2017;10:895–907.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:728–34.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng WJ, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Watanabe T, Tobinai K, Matsumoto M, Suzuki K, Sunami K, Ishida T, et al. A phase 1/2 study of carfilzomib in Japanese patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2016;172:745–56.
Iida S, Watanabe T, Matsumoto M, Suzuki K, Sunami K, Ishida T, et al. Carfilzomib monotherapy in Japanese patients with relapsed or refractory multiple myeloma: a phase 1/2 study. Cancer Sci. 2019;110:2924–32.
Suzuki K, Ri M, Chou T, Sugiura I, Yakezako N, Sunami K, et al. Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: a phase 1 study in Japan. Cancer Sci. 2017;108:461–8.
Iida S, Tobinai K, Taniwaki M, Shumiya Y, Nakamura T, Chou T. Phase I dose escalation study of high dose carfilzomib monotherapy for Japanese patients with relapsed or refractory multiple myeloma. Int J Hematol. 2016;104:596–604.
Dimopoulos MA, Stewart AK, Masszi T, Špička I, Oriol A, Hájek R, et al. Carfilzomib, lenalidomide, and dexamethasone in patients with relapsed multiple myeloma categorised by age: secondary analysis from the phase 3 ASPIRE study. Br J Haematol. 2017;177:404–13.
Dimopoulos MA, Stewart AK, Masszi T, Špička I, Oriol A, Hájek R, et al. Carfilzomib-lenalidomide-dexamethasone vs lenalidomide-dexamethasone in relapsed multiple myeloma by previous treatment. Blood Cancer J. 2017;7:e554.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Suzuki K, Sunami K, Ohashi K, Iida S, Mori T, Handa H, et al. Randomized phase 3 study of elotuzumab for relapsed or refractory multiple myeloma: ELOQUENT-2 Japanese patient subanalysis. Blood Cancer J. 2017;7:e540.
Richardson PG, Xie W, Jagannath S, Jakubowiak A, Lonial S, Raje NS, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–9.
Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–9.
Dytfeld D, Jasielec J, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162–4.
Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1:746–54.
Acknowledgements
We thank all study participants and their families. We thank all study sites and investigators. We would also like to thank the medical consultant, Dr. Hirokazu Murakami (Gunma University Graduate School of Health Science, Maebashi), and Dr. Chihiro Shimazaki (Japan Community Health Care Organization Kyoto-Kuramaguchi Medical Center, Kyoto), Dr. Masahiro Kizaki (Saitama Medical Center, Saitama Medical University, Saitama), Dr. Takao Katoh (International University of Health and Welfare, Mita Hospital, Tokyo), Dr. Masahiro Endo (Shizuoka Cancer Center, Nagaizumi), and Dr. Terufumi Kato (Kanagawa Cancer Center, Yokohama) for their review of the clinical data as members of the Efficacy and Safety Evaluation Committee. We also acknowledge the statistical support of Naokazu Gion and Toshiaki Ozaki (Ono Pharmaceutical, Osaka) and the critical review of the manuscript by Amgen (Thousand Oaks). We thank Susan Cottrell, PhD, and Keyra Martinez Dunn, MD, of Edanz Medical Writing for providing medical writing support, which was funded by Ono Pharmaceutical through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
IS, KS, MR, TC, NT, KS, T Ishida, T Izumi, SO, and SI received grants from Ono Pharmaceutical during the course of this study. MR also received grants and personal fees from Bristol-Myers Squibb, Celgene, Janssen Pharmaceutical, Kyowa Hakko Kirin, Ono Pharmaceutical, and Takeda Pharmaceutical, outside the submitted work, and personal fees from Novartis and Sanofi, outside the submitted work. TC also received personal fees from Ono Pharmaceutical during the course of the study and personal fees from Janssen Pharmaceutical, Ono Pharmaceutical, and Takeda Pharmaceutical, outside the submitted work. NT also received personal fees from Ono Pharmaceutical during the course of this study. KS also received personal fees from Ono Pharmaceutical during the course of this study; grants from AbbVie, Alexion Pharma, Daiichi Sankyo, GlaxoSmithKline, Janssen Pharmaceutical, MSD, Novartis, and Sanofi, outside the submitted work; and grants and personal fees from Bristol-Myers Squibb, Celgene, and Takeda Pharmaceutical, outside the submitted work. T Ishida also received personal fees from Celgene, Janssen Pharmaceutical, Ono Pharmaceutical, and Takeda Pharmaceutical, outside the submitted work. SI also received personal fees from Ono Pharmaceutical during the course of this study; grants and personal fees from Bristol-Myers Squibb, Celgene, Janssen Pharmaceutical, Novartis, and Takeda Pharmaceutical, outside the submitted work; and grants from AbbVie, Chugai Pharmaceutical, Daiichi Sankyo, Gilead Pharmaceutical, Kyowa Hakko Kirin, MSD, and Sanofi, outside the submitted work. YS is an employee of Ono Pharmaceutical. The study was designed under the responsibility of Ono Pharmaceutical, in conjunction with the steering committee; the study was funded by Ono Pharmaceutical; carfilzomib was provided by Ono Pharmaceutical; Ono Pharmaceutical collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sugiura, I., Suzuki, K., Ri, M. et al. Final results of a phase I study of carfilzomib, lenalidomide, and dexamethasone for heavily pretreated multiple myeloma. Int J Hematol 111, 57–64 (2020). https://doi.org/10.1007/s12185-019-02754-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02754-3