Abstract
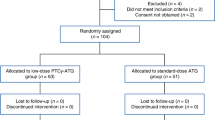
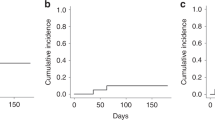
This phase I study was designed for graft-versus-host disease (GVHD) prophylaxis including bortezomib in allogeneic hematopoietic cell transplantation (allo-HCT) from human leukocyte antigen (HLA)-mismatched unrelated donors in Japanese patients. Patients were administered bortezomib on days 1, 4, and 7, with short-term methotrexate and tacrolimus. Three bortezomib dose levels were prepared (1.0, 1.3, and 1.5 mg/m2). A dose of 1.3 mg/m2 was planned for administration to the initial six patients, and was adjusted if dose-limiting toxicity developed. Five of six patients enrolled for the initial dose had bone marrow donors. Two cases had single-antigen and single-allele mismatches; four had single-antigen mismatch at the A, B, C, and/or DRB1 loci in the GVH direction. All patients achieved neutrophil engraftment and complete donor chimerism. Three patients developed grade II acute GVHD, and none developed grade III–IV GVHD or any dose-limiting toxicity attributable to bortezomib by day 100. Two patients developed late-onset acute GVHD, and two developed chronic GVHD, but all cases were manageable. All patients were alive without relapse after a median follow-up period of 52 months. The optimal dose of bortezomib was determined to be 1.3 mg/m2. Prophylaxis against GVHD using a regimen including bortezomib thus seems feasible for HLA-mismatched unrelated allo-HCT.

Similar content being viewed by others
References
Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–702.
Woolfrey A, Lee SJ, Gooley TA, Malkki M, Martin PJ, Pagel JM, et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: role of cell source and disease risk category. Biol Blood Marrow Transplant. 2010;16:1382–7.
Verneris MR, Lee SJ, Ahn KW, Wang HL, Battiwalla M, Inamoto Y, et al. HLA mismatch is associated with worse outcomes after unrelated donor reduced-intensity conditioning hematopoietic cell transplantation: an analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2015;21:1783–9.
Yokoyama H, Kanda J, Fuji S, Kim SW, Fukuda T, Najima Y, et al. Impact of human leukocyte antigen allele mismatch in unrelated bone marrow transplantation with reduced-intensity conditioning regimen. Biol Blood Marrow Transplant. 2017;23:300–9.
Kanda J. Effect of HLA mismatch on acute graft-versus-host disease. Int J Hematol. 2013;98:300–8.
Mehta RS, Saliba RM, Chen J, Rondon G, Hammerstrom AE, Alousi A, et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br J Haematol. 2016;173:444–55.
Jorge AS, Suárez-Lledó M, Pereira A, Gutierrez G, Fernández-Avilés F, Rosiñol L, et al. Single antigen-mismatched unrelated hematopoietic stem cell transplantation using high-dose post-transplantation cyclophosphamide is a suitable alternative for patients lacking HLA-matched donors. Biol Blood Marrow Transplant. 2018;24:1196–202.
Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolaños-Meade J, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1:288–92.
Soltermann Y, Heim D, Medinger M, Baldomero H, Halter JP, Gerull S, et al. Reduced dose of post-transplantation cyclophosphamide compared to ATG for graft-versus-host disease prophylaxis in recipients of mismatched unrelated donor hematopoietic cell transplantation: a single-center study. Ann Hematol. 2019;98:1485–93.
Nencioni A, Schwarzenberg K, Brauer KM, Schmidt SM, Ballestrero A, Grünebach F, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108:551–8.
Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, Carvajal-Vergara X, Carvajal-Vergara X, Mateos J, Vidriales B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–83.
Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–59.
Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–9.
Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–8.
Koreth J, Kim HT, Lange PB, Bindra B, Reynolds CG, Chammas MJ, et al. A bortezomib-based regimen offers promising survival and graft-versus-host disease prophylaxis in myeloablative hla-mismatched and unrelated donor transplantation: a phase II trial. Biol Blood Marrow Transplant. 2015;21:1907–13.
Koreth J, Kim HT, Lange PB, Poryanda SJ, Reynolds CG, Rai SC, et al. Bortezomib-based immunosuppression after reduced-intensity conditioning hematopoietic stem cell transplantation: randomized phase II results. Haematologica. 2018;103:522–30.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versushost disease: I Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Kawase T, Morishima Y, Matsuo K, Kashiwase K, Inoko H, Saji H, Japan Marrow Donor Program et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. 2007;110:2235–41.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–e143143.
Nagafuji K, Matsuo K, Teshima T, Mori S, Sakamaki H, Hidaka M, et al. Peripheral blood stem cell versus bone marrow transplantation from HLA-identical sibling donors in patients with leukemia: a propensity score-based comparison from the Japan Society for Hematopoietic Stem Cell Transplantation registry. Int J Hematol. 2010;91:855–64.
Savani BN, Labopin M, Blaise D, Niederwieser D, Ciceri F, Ganser A, et al. Peripheral blood stem cell graft compared to bone marrow after reduced intensity conditioning regimens for acute leukemia: a report from the ALWP of the EBMT. Haematologica. 2016;101:256–62.
Ganetsky A, Shah A, Miano TA, Hwang WT, He J, Loren AW, et al. Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:568–72.
Morishima Y, Kawase T, Malkki M, Morishima S, Spellman S, Kashiwase K, et al. Significance of ethnicity in the risk of acute graft-versus-host disease and leukemia relapse after unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1197–203.
Kanda J, Brazauskas R, Hu ZH, Kuwatsuka Y, Kuwatsuka Y, Nagafuji K, Kanamori H, et al. Graft-versus-host disease after HLA-matched sibling bone marrow or peripheral blood stem cell transplantation: comparison of North American Caucasian and Japanese Populations. Biol Blood Marrow Transplant. 2016;22:744–51.
Acknowledgements
We thank all the participating patients and the medical staff who provided care for these patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Nakane received research funding from Pfizer Inc. Y. Nakashima received research funding from Astellas. M. Hino received research funding from Astellas and Pfizer Inc. and a speaker’s honoraria from Pfizer Inc. H. Nakamae received research funding from Astellas and a speaker’s honoraria from Pfizer Inc. All other authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakane, T., Okamura, H., Tagaito, Y. et al. Phase I study of graft-versus-host disease prophylaxis including bortezomib for allogeneic hematopoietic cell transplantation from unrelated donors with one or two HLA loci mismatches in Japanese patients. Int J Hematol 110, 736–742 (2019). https://doi.org/10.1007/s12185-019-02743-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02743-6