Abstract
Chronic lymphocytic leukemia (CLL) can trigger autoimmune phenomena, with immune thrombocytopenia (ITP) the most common presentation. Upon cessation of CLL therapy, including ibrutinib, autoimmune flares can occur. In a 68-year-old man with CLL, ibrutinib was held for 2 weeks prior to elective shoulder surgery. Eleven days after stopping therapy, he presented with a purpuric rash on his right hip, buttock, and lower extremities. He experienced two episodes of seizure activity while hospitalized. MRI brain demonstrated patchy areas of altered signal involving deep white matter and sub-cortical white matter structures concerning for cerebral vasculitis. Although there was no evidence of hemolysis, serum cold agglutinin titer was elevated at > 1:512 and cryoglobulin levels were positive at 36%. He was diagnosed with type I cryoglobulinemia and treated with rituximab, plasmapheresis, methylprednisolone, and ibrutinib was restarted. This regimen resolved his symptoms. A rare complication of CLL is the production of cryoglobulins, which can present at initial diagnosis or in relapsed disease. Our case demonstrates that the cessation of ibrutinib therapy, even for a short time, can precipitate complications. To our knowledge, we report the first case of a patient with well-controlled CLL who rapidly developed cryoglobulinemic vasculitis after stopping ibrutinib therapy.
Similar content being viewed by others
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the western hemisphere with an estimated 15,000 newly diagnosed cases every year [1]. In 10–25% of cases autoimmune complications occur, the most common being warm autoimmune hemolytic anemia (AIHA) [2]. Less common in CLL is the production of antibodies that agglutinate at cold temperatures (cold agglutinins) which can present at initial presentation or in relapsed disease [3, 4]. In contrast with warm autoantibodies, which are typically IgG and, therefore, are IgG ± C3+ on direct Coomb’s testing, these are usually IgM antibodies that are IgG−/C3+ and are associated with acrocyanosis. Even rarer is when these antibodies not only agglutinate at cold temperatures, but cryoprecipitate as well. In this case, hemolysis would be expected and also the features of a cryoglobulinemia, which include cutaneous purpura that are relatively benign to renal involvement with life-threatening vasculitis.
Over the past few years, the armamentarium to treat CLL has expanded with first-line CLL therapy now including oral ibrutinib, a covalent inhibitor of Bruton’s tyrosine kinase [5]. CLL can trigger autoimmune complications, and in addition, agents used to treat CLL such as the alkylating agent fludarabine [6] can cause autoimmune phenomena, with immune thrombocytopenia (ITP) being the most common presentation [7]. During clinical trials of ibrutinib, autoimmune complications [8] were not reported; however, reports of autoimmune flares on stopping ibrutinib have emerged [9]. To our knowledge, we report the first case of a patient with well-controlled CLL who, after stopping ibrutinib therapy, developed an antibody with properties of a cold agglutinin and cryoglobulin that caused cryglobulinemic vasculitis.
Case presentation
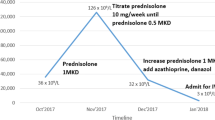
A 68-year-old male with 2-year history of CLL, on ibrutinib therapy, was scheduled for an elective knee surgery and was recommended to hold ibrutinib for 2 weeks prior to surgery. Eleven days after holding therapy, he presented with right flank pain and a rash. Physical exam demonstrated a petechial rash in the right hip, buttock, and lower extremities. Initial blood work demonstrated a white blood cell count of 11,700/mm3, hemoglobin of 15.9 g/dl, and platelets of 78,000/mm3. His lymphocyte percent was 41%. Computed tomography (CT) of abdomen/pelvis demonstrated no obvious findings to explain presenting symptoms. An infectious work up, including blood cultures, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C were negative. While at the hospital, he experienced two episodes of focal right-sided seizures lasting 30 min each. He was started on levetiracetam and underwent a magnetic resonance imaging (MRI) of the brain, which demonstrated extensive patchy areas of altered signal involving deep white matter and sub-cortical white matter structures concerning for cerebral vasculitis. His original rash progressed in size and developed an ecchymotic appearance with spread to the thorax and bilateral upper extremities. Further lab work was negative for disseminated intravascular coagulopathy (DIC) and antinuclear antibody (ANA), but did show low C3 and C4 levels. Peripheral blood smear was notably absent of plasmacytoid cells and any evidence of transformation. The clinical pathology department noted clumping on the smear, at which time a cold agglutinin titer was checked. There was no evidence of hemolysis, but the titer was elevated at 1:512 (normal titer: < 1:64). Serum immunoglobulin levels were elevated with an IgM of 559 mg/dl (normal: 40–230 mg/dl) with serum protein electrophoresis demonstrating a monoclonal IgM kappa band. Given his clinical picture, a serum cryoglobulin screen was sent which was positive. This led to the conclusion that the antibody was behaving as both a cold agglutinin and cryoglobulin. He was given rituximab (375 mg/m2) and was transferred to our center for urgent plasmapheresis.
On transfer, the skin findings included tender, large retiform purpuric patches overlying the abdomen, lateral back, buttock, hips, chest, shoulders, and arms, with flaccid bullae within the center of the patches (Fig. 1a). The patient also had multiple purpuric patches scattered on the bilateral lower legs and feet along with dusky purple discoloration of the right 1st and 3rd toes (Fig. 1b). He was in moderate distress from pain at rest. His complete blood count panel and peripheral smear was noted to have significant clumping further supporting a diagnosis of cold agglutinins. He was started on therapeutic plasma exchange with concurrent high-dose corticosteroids (methylprednisolone 125 mg intravenously twice daily) for the treatment of cryoglobulinemic vasculitis.
a Purpuric rash with central bullae overlying the right hip and buttock. b Purple discoloration of the first and third toes with tense bullae. These classic skin findings of purpura along with laboratory values with elevated cold agglutinin titer, low C3 and C4, and elevated monoclonal IgM Kappa levels are supportive of diagnosis of type 1 cryoglobulinemia
He had a remarkable improvement in symptoms within 24 h of initiating plasma exchange (Fig. 2a, b). Importantly, he was restarted on his prior dose of ibrutinib at 420 mg daily. He completed two additional sessions of plasma exchange over the following week and was continued on weekly rituximab for four total cycles with a steroid taper over the course of 1 month. He continued to improve with undetectable repeat cryoglobulin levels with no clumping on the peripheral smear. He was discharged on 420 mg of ibrutinib daily with good control of his disease. He is now 6 month post-treatment and has no evidence of recurrent vasculitis.
Discussion
Cryoglobulins are proteins that are soluble at 37 °C and precipitate at lower temperatures. They are classified into three subgroups based on the Brouet classification, which uses immunochemical composition to determine the cause of cryoglobulinemia [10]. Monoclonal, type I, cryoglobulins are comprised of a single monoclonal immunoglobulin (Ig), most commonly IgM, and account for 10–15% of patients with cryoglobulinemia with the most common etiology being an underlying malignancy [11,12,13]. Lymphoproliferative disorders, such as Waldenstrom’s macroglobulinemia, Multiple myeloma, and CLL, have been classically defined as the underlying malignant processes that lead to type I cryoglobulinemia [14,15,16]. However, even within this cohort, CLL forms a small percentage of cases. In one study of 86 patients with cryoglobulinemia, only 3 patients had CLL, none of whom had type I cryoglobulinemia [17]. Another series of 64 patients with type I cryoglobulinemia included just two patients with CLL [18].
Cryoglobulinemia can be life threatening due to the extent of cutaneous and visceral involvement [19]. While vasculitis and renal impairment are the most common presenting features of the disease, neurological complications may also occur. This is usually in the form of distal sensory or sensory-motor polyneuropathy but may also include pure sensory mononeuropathy and mononeuritis multiplex [18]. Central nervous system (CNS) involvement is very rare, even more so in the setting of type 1 disease. In the large French nationwide CryoVas survey of 471 patients with cryoglobulinemia, 11 patients had CNS manifestations, none of whom had type I disease [19]. Our patient’s initial presentation was non-specific with rash and flank pain. MRI brain with findings concerning for vasculitis was the first clue definitively suggesting vasculitis and, therefore, cryoglobulinemia as the culprit of his symptoms (Fig. 3). The development of CNS complications from cryoglobulinemia is noteworthy and clinicians should have a high degree of suspicion for cryoglobulinemia in patients with the appropriate risk factors and clinical picture with otherwise unexplained CNS complications.
Autoimmunity in CLL predominantly targets blood constituents. Autoantibodies in CLL are polyclonal, differing in specificity and isotype from the immunoglobulins secreted directly by CLL cells [20]. As a result, non-malignant B cells are believed to be responsible for producing auto-antibodies. Another possible explanation is the presence of autoreactive T cells which are normally suppressed by regulatory T cells (T-regs). T-regs can be ineffective in CLL and are suppressed in patients treated with alkylating agents, especially busulfan, providing a pathway for autoimmune disease in the setting of treatment.
Ibrutinib is an oral covalent inhibitor of Bruton’s tyrosine kinase (BTK) which has demonstrated efficacy in prolonging progression free survival (PFS) and overall survival (OS) in CLL, including those with relapsed/refractory disease [21, 22]. Inhibition of BTK can have pleiotropic effects on different components of the immune system [23].
In our case, what is remarkable is the emergence of cryoglobinemia upon discontinuation of therapy with no prior history of the same. The cryoglobulins were IgM in nature, suggesting that a single clone escaped inhibition and propagated rapidly in the absence of ibrutinib. Subsequent therapy with rituximab, steroids, and initiation of ibrutinib likely suppressed the clone. Sato et al. reported a similarly rapid development of autoimmune phenomena in a patient upon cessation of ibrutinib; however, their patient experienced ITP [9]. Our case is an example of an even less common autoimmune complication of CLL arising upon discontinuation of therapy with ibrutinib.
There have been reports in the literature of ibrutinib being used to treat autoimmune phenomena such as AIHA [24]. In this case, we believe stopping ibrutinib unmasked the underlying disease—possibly even accelerating it. Our case illustrates the rapidity with which complications can occur in CLL, even in an individual with otherwise well-controlled disease. It simultaneously reaffirms the effectiveness with which ibrutinib can control the disease. Further research is warranted to determine relevant risk factors of patients prone to developing autoimmune phenomena, as well as the most appropriate treatment for when complications do occur.
References
Jemal A, Siegal R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
Zent CS, Kay NE. Autoimmune complications in chronic lymphocytic leukaemia (CLL). Best Pract Res Clin Haematol. 2010;23(1):47–59.
Ruzickova S, Pruss A, Odendahl M, Wolbart K, Burmester GR, Scholze J, et al. Chronic lymphocytic leukemia preceded by cold agglutinin disease: intraclonal immunoglobulin light-chain diversity in V(H)4-34 expressing single leukemic B cells. Blood. 2002;100(9):3419–22.
Berentsen S, Bø K, Shammas FV, Myking AO, Ulvestad E. Chronic cold agglutinin disease of the “idiopathic” type is a premalignant or low-grade malignant lymphoproliferative disease. APMIS. 1997;105(5):354–62.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37.
Lewis FB, Schwartz RS, Dameshek W. X-radiation and alkylating agents as possible “trigger” mechanisms in the autoimmune complications of malignant lymphophroliferative disease. Clin Exp Immunol. 1966;1(1):3–11.
Barcellini W, Capalbo S, Agostinelli RM, Mauro FR, Ambrosetti A, Calori R, et al. Relationship between autoimmune phenomena and disease stage and therapy in B-cell chronic lymphocytic leukemia. Haematologica. 2006;91(12):1689–92.
Rogers KA, Ruppert AS, Bingman A, Andritsos LA, Awan FT, Blum KA, et al. Incidence and description of autoimmune cytopenias during treatment with ibrutinib for chronic lymphocytic leukemia. Leukemia. 2016;30(2):346–50.
Sato R, Jacob J, Gaballa S. Rapid flare of immune thrombocytopenia after stopping ibrutinib in a patient with chronic lymphocytic leukemia. Leuk Lymphoma. 2017;59(7):1–4.
Brouet J-C, Clauvel J-P, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins: a report of 86 cases. Am J Med. 1974;57(5):775–88.
Meltzer M, Franklin EC. Cryoglobulinemia—a study of twenty-nine patients. Am J Med. 1966;40(6):828–36.
Morra E. Cryoglobulinemia. Hematol Am Soc Hematol Educ Progr 2005;1(1):368–72.
Xu W, Wang Y-H, Fan L, Fang C, Zhu DX, Wang DM, et al. Prognostic significance of serum immunoglobulin paraprotein in patients with chronic lymphocytic leukemia. Leuk Res. 2011;35(8):1060–5.
Michael AB, Lawes M, Kamalarajan M, Huissoon A, Pratt G. Cryoglobulinaemia as an acute presentation of waldenstrom’s macroglobulinaemia. Br J Haematol. 2004;124(5):565.
Payet J, Livartowski J, Kavian N, Chandesris O, Dupin N, Wallet N, et al. Type I cryoglobulinemia in multiple myeloma, a rare entity: analysis of clinical and biological characteristics of seven cases and review of the literature. Leuk Lymphoma. 2013;54(4):767–77.
Néel A, Perrin F, Decaux O, Dejoie T, Tessoulin B, Halliez M, et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am J Hematol. 2014;89(2):156–61.
Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57(5):775–88.
Terrier B, Karras A, Kahn J-E, Guenno GL, Marie I, Benarous L, et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (Baltimore). 2013;92(2):61–8.
Terrier B, Cacoub P. Cryoglobulinemia vasculitis: an update. Curr Opin Rheumatol. 2013;25(1):10–8.
Dearden C. Disease-specific complications of chronic lymphocytic leukemia. Hematology. 2008;2008(1):450–6.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
O’Brien S, Jones JA, Coutre SE, Mato AR, Hillman P, Tam C, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–18.
Montillo M, O’Brien S, Tedeschi A, Byrd JC, Dearden C, Gill D, et al. Ibrutinib in previously treated chronic lymphocytic leukemia patients with autoimmune cytopenias in the RESONATE study. Blood Cancer J. 2017;7(2):e524.
Manda S, Dunbar N, Marx-Wood CR, Danilov AV. Ibrutinib is an effective treatment of autoimmune haemolytic anaemia in chronic lymphocytic leukaemia. Br J Haematol. 2015;170(5):734–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Wright, N., Voshtina, E., George, G. et al. Cryoglobulinemic vasculitis with interruption of ibrutinib therapy for chronic lymphocytic leukemia (CLL). Int J Hematol 110, 751–755 (2019). https://doi.org/10.1007/s12185-019-02729-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02729-4