Abstract
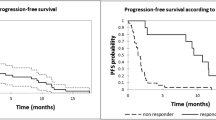
Daratumumab is a human anti-CD38 monoclonal antibody used in the treatment of refractory and relapsed multiple myeloma. We investigated the efficacy and safety of daratumumab therapy in a real-world setting. Ninety-nine Hungarian patients were included; 48 received monotherapy, while lenalidomide and bortezomib combinations were administered in 29 and 19 cases, respectively. Overall response rate was assessable in 88 patients, with 12 complete, 10 very good partial, 34 partial, and seven minor responses. At a median duration of follow-up of 18.6 months, median progression-free survival (PFS) among all patients was 17.0 months. These values were inferior in the bortezomib combination and monotherapy groups. Patients with early-stage disease (ISS1) had better survival results than those with stage 2 or 3 myeloma (p = 0.009). Heavily pretreated patients had inferior PFS compared to those with 1–3 therapies (p = 0.035). Patients with impaired renal function had PFS results comparable with those having no kidney involvement. There were 10 fatal infections, and the most frequent adverse events were mild infusion-associated reactions and hematologic toxicities. Our results confirm that daratumumab is an effective treatment option for relapsed/refractory MM with an acceptable safety profile in patients with normal and impaired renal function.






Similar content being viewed by others
References
Raza S, Safyan RA, Rosenbaum E, Bowman A, Lentzsch S. Optimizing current and emerging therapies in multiple myeloma: a guide for the haematologist. Ther Adv Hematol. 2017;8:55–70.
Morandi F, Horenstein AL, Costa F, Guiliani N, Pistoia V, Malavasi F. CD38: a target for immunotherapeutic approaches in multiple myeloma. Front Immunol. 2018;9:2722. https://doi.org/10.3389/fimmu.2018.02722 (eCollection).
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8.
Van der Weer MS, De Weers M, Van Kessel B, Bakker JM, Wittebol S, Parren PW, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Hematologica. 2011;96:284–90.
Nijhof IS, Groen RW, Noort WA, van Kessel B, de Jong-Korlaar R, Bakker J, et al. Preclinical evidence for the therapeutic potential of CD28-targeted immune-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21:2802–10.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomized, phase 2 trial. Lancet. 2016;387:1551–60.
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128:37–44.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66.
Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–87.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma. Haematologica. 2018;103(12):2088–96.
Minarik J, Pour L, Maisnar V, Spicka I, Jungova A, Jelinek T, et al. Single agent daratumumab in advanced multiple myeloma possesses significant efficacy even in an unselected “real-world” population. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018. https://doi.org/10.5507/bp.2018.064.
Jullien M, Trudel S, Tessoulin B, Mahé B, Dubruille V, Blin N, et al. Single-agent daratumumab in very advanced relapsed and refractory multiple myeloma patients: a real-life single-center retrospective study. Ann Hematol. 2019. https://doi.org/10.1007/s00277-019-03655-5.
Richardson PG, San Miguel JF, Moreau P, Hajek R, Dimopoulos MA, Laubacj JP, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109.
Funding
No funding is applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Lovas, S., Varga, G., Farkas, P. et al. Real-world data on the efficacy and safety of daratumumab treatment in Hungarian relapsed/refractory multiple myeloma patients. Int J Hematol 110, 559–565 (2019). https://doi.org/10.1007/s12185-019-02715-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02715-w