Abstract
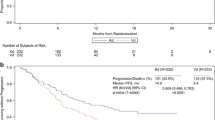
Carfilzomib is an irreversible proteasome inhibitor used for the treatment of relapsed and/or refractory multiple myeloma (RRMM). We evaluated the efficacy and safety of carfilzomib in subgroups of Asian patients in the randomized phase 3 ENDEAVOR and A.R.R.O.W. trials. In ENDEAVOR, patients received carfilzomib twice-weekly (56 mg/m2) plus dexamethasone (Kd; n = 56) or bortezomib plus dexamethasone (Vd; n = 57). In A.R.R.O.W., patients received carfilzomib once-weekly (70 mg/m2, n = 30) or twice-weekly (27 mg/m2, n = 15) plus dexamethasone. Median progression-free survival (PFS) among Asian patients in ENDEAVOR was longer with Kd than with Vd (14.9 versus 8.8 months; HR 0.599); the overall response rate (ORR) was 80.4% versus 70.2%. Median overall survival (Kd versus Vd) was 47.6 versus 38.8 months (HR 0.856). Median PFS among Asian patients in A.R.R.O.W. was longer for once-weekly versus twice-weekly Kd (16.0 versus 8.4 months; HR 0.628); ORR was 76.7% versus 53.3%. Rates of grade ≥ 3 adverse events were 89.1% (Kd) and 89.5% (Vd) in ENDEAVOR, and 76.6% (once-weekly Kd) versus 73.3% (twice-weekly Kd) in A.R.R.O.W. Overall, carfilzomib had a favorable benefit-risk profile across both dosing regimens [once-weekly (Kd 70 mg/m2) and twice-weekly (Kd 56 mg/m2)] in Asian patients with RRMM, which was consistent with the results of both parent studies.
Trial registration ClinicalTrials.gov: NCT01568866, NCT02412878.



Similar content being viewed by others
References
Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. Global burden of multiple myeloma: a systematic analysis for the Global Burden of Disease study 2016. JAMA Oncol. 2018;4:1221–7.
Cornell RF, Kassim AA. Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity. Bone Marrow Transpl. 2016;51:479–91.
Kim K, Lee JH, Kim JS, Min CK, Yoon SS, Shimizu K, et al. Clinical profiles of multiple myeloma in Asia—an Asian Myeloma Network study. Am J Hematol. 2014;89:751–6.
Hong J, Lee JH. Recent advances in multiple myeloma: a Korean perspective. Korean J Intern Med. 2016;31:820–34.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Tan D, Lee JH, Chen W, Shimizu K, Hou J, Suzuki K, et al. Recent advances in the management of multiple myeloma: clinical impact based on resource-stratification. Consensus statement of the Asian Myeloma Network at the 16th International Myeloma Workshop. Leuk Lymphoma. 2018;59:1–13.
Suzuki K, Dimopoulos MA, Takezako N, Okamoto S, Shinagawa A, Matsumoto M, et al. Daratumumab, lenalidomide, and dexamethasone in East Asian patients with relapsed or refractory multiple myeloma: subgroup analyses of the phase 3 POLLUX study. Blood Cancer J. 2018;8:41.
Hou J, Jin J, Xu Y, Wu D, Ke X, Zhou D, et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study. J Hematol Oncol. 2017;10:137.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34.
Kyprolis® (carfilzomib). Full prescribing information. Thousand Oaks: Amgen Inc.; 2018.
Kyprolis (carfilzomib). Summary of product characteristics. Breda: Amgen Europe; 2018.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng WJ, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Moreau P, Mateos MV, Berenson JR, Weisel K, Lazzaro A, Song K, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19:953–64.
Mikhael J. Management of carfilzomib-associated cardiac adverse events. Clin Lymphoma Myeloma Leuk. 2016;16:241–5.
Watanabe T, Tobinai K, Matsumoto M, Suzuki K, Sunami K, Ishida T, et al. A phase 1/2 study of carfilzomib in Japanese patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2016;172:745–56.
Iida S, Tobinai K, Taniwaki M, Shumiya Y, Nakamura T, Chou T. Phase I dose escalation study of high dose carfilzomib monotherapy for Japanese patients with relapsed or refractory multiple myeloma. Int J Hematol. 2016;104:596–604.
Maruyama D, Tobinai K, Chou T, Taniwaki M, Shumiya Y, Iida S. Weekly carfilzomib and dexamethasone in Japanese patients with relapsed or refractory multiple myeloma: a phase 1 and PK/PD trial. Cancer Sci. 2018;109:3245–52.
Suzuki K, Ri M, Chou T, Sugiura I, Takezako N, Sunami K, et al. Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: a phase 1 study in Japan. Cancer Sci. 2017;108:461–8.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Acknowledgements
The authors thank the patients and sites for their participation in the ENDEAVOR and A.R.R.O.W. trials. Jesse Potash, PhD (Amgen Inc.) and Meghan Johnson, PhD (Complete Healthcare Communications, LLC, an ICON plc company, North Wales, PA, USA), whose work was funded by Amgen Inc., provided medical writing assistance in the preparation of this manuscript.
Funding
The ENDEAVOR and A.R.R.O.W. studies were sponsored and funded by Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MAD reports receiving honoraria and consulting and/or advisory role fees from Celgene, Janssen, Amgen Inc., Novartis, and Takeda, and research funding from Genesis Pharma. PM reports receiving honoraria from and advisory board participation with Amgen Inc., Celgene, Takeda, Janssen, and AbbVie. SI reports receiving research grants and consulting fees from Ono Pharmaceutical Co., Ltd., Janssen, Celgene, Takeda, Bristol-Myers Squibb, and Novartis; and research grants from Chugai, Teijin Pharma, Astellas, Toyama Chemical, Eli Lilly, Kyowa Hakko Kirin, Sanofi, AbbVie, Merck Sharp & Dohme, and Daiichi Sanko. WJC reports receiving research grants from Celgene, Janssen, Novartis, Takeda, and Amgen Inc.; and consulting fees from Celgene, Janssen, Novartis, Takeda, and Amgen Inc. AZ-K, MAS, JL, and MH are employees of and own stock in Amgen Inc. S-YH, NT, and JHL have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Dimopoulos, M.A., Moreau, P., Iida, S. et al. Outcomes for Asian patients with multiple myeloma receiving once- or twice-weekly carfilzomib-based therapy: a subgroup analysis of the randomized phase 3 ENDEAVOR and A.R.R.O.W. Trials. Int J Hematol 110, 466–473 (2019). https://doi.org/10.1007/s12185-019-02704-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02704-z