Abstract
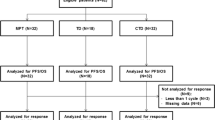
We conducted a phase I study to determine the recommended dose of thalidomide combined with melphalan plus prednisolone (MPT) and a phase II study evaluating the efficacy and safety of this MPT regimen in transplant-ineligible Japanese patients with untreated multiple myeloma. The recommended dose was determined to be 100 mg/day in the phase I study. In the phase II, randomized, double-blind, parallel-group study, patients were allocated to either MPT (n = 52) or MP (n = 51), with 21 and 29 patients completing the study, respectively. Overall response rate, the primary endpoint, was significantly higher in the MPT [40.4% (21/52 patients), 95% confidence interval (CI) 27.0–54.9%] than in the MP [19.6% (10/51 patients), 95% CI 9.8–33.1%] group (P = 0.022). Time to response was also significantly shorter in the MPT group. Incidences of hematological toxicities were similar in the two groups, suggesting that addition of thalidomide did not increase hematological toxicity. Although incidences of some non-hematological toxicities tended to be higher in the MPT group, the low incidence of ≥ Grade 3 toxicities suggests that MPT therapy was well tolerated. These results support the safety and efficacy of MPT therapy in untreated Japanese multiple myeloma patients.




Similar content being viewed by others
References
Alexanian R, Haut A, Khan AU, Lane M, McKelvey EM, Migliore PJ, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208:1680–5.
Mateos MV, Hernández JM, Hernández MT, Gutiérrez NC, Palomera L, Fuertes M, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–72.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17.
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Intergroupe Francophone du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomized trial. Lancet. 2007;370:1209–18.
Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM01/01 trial. J Clin Oncol. 2009;27:3664–70.
Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Italian Multiple Myeloma Network, GIMEMA. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomized controlled trial. Lancet. 2006;367:825–31.
Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, Dutch-Belgium Cooperative Group HOVON, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 study. J Clin Oncol. 2010;28:3160–6.
Waage A, Gimsing P, Fayers P, Abildgaard L, Ahlberg L, Bjorkstrand B, Nordic Myeloma Study Group, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–12.
Beksac M, Haznedar R, Firatli-Tuglular T, Ozdougu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22.
Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–17.
Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksac M, Nordic Myeloma Study Group; Italian Multiple Myeloma Network; Turkish Myeloma Study Group; Hemato-Oncologie voor Volwassenen Nederland; Intergroupe Francophone du Myélome; European Myeloma Network, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239–47.
Ludwig H, Sonneveld P, Davies F, Bladé J, Boccadoro M, Cavo M, et al. European perspective on multiple myeloma treatment strategies in 2014. Oncologist. 2014;19:829–44.
Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv52–61.
Iida S, Ishida T, Murakami H, Ozaki S, Abe M, Hata H, et al. JSH practical guidelines for hematological malignancies, 2018: III. Myeloma-1 Multiple myeloma (MM). Int J Hematol. 2019;109:509–38.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57.
Common Terminology Criteria for Adverse Events v4.0 Japanese version (CTCAE)-JCOG (in Japanese). http://www.jcog.jp/doctor/tool/CTCAEv4J_20170912_v20_1.pdf. Accessed 24 Apr 2019.
Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
Palumbo A, Waage A, Hulin C, Beksac M, Zweegman S, Gay F, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: a meta-analysis of data from individual patients in six randomized trials. Haematologica. 2013;98:87–94.
Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–9.
Guidelines for Diagnosis and Treatment of Multiple Myeloma. 2nd ed. Japanese Society of Myeloma, editors. Tokyo: Bunkodo; 2008 (in Japanese).
Handa H, Murakami H, Kosugi H, Igarashi T, Taniwaki M, Matsumoto M, et al. Melphalan/prednisolone/zoledronic acid therapy in newly diagnosed Japanese patients with multiple myeloma. In: 14th International Myeloma Workshop, Abstract. 2013. S136.
Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600.
Larocca A, Palumbo A. How I treat fragile myeloma patients. Blood. 2015;126:2179–85.
Iida S, Wakabayashi M, Tsukasaki K, Miyamoto K, Maruyama D, Yamamoto K, et al. Bortezomib plus dexamethasone vs thalidomide plus dexamethasone for relapsed or refractory multiple myeloma. Cancer Sci. 2018;109:1552–61.
Murakami H, Kasamatsu T, Murakami J, Kiguchi T, Kanematsu T, Ogawa D, et al. Thalidomide maintenance therapy in Japanese myeloma patients: a multicenter, phase II clinical trial (COMET study). Int J Hematol. 2019;109:409–17.
Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E, et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34:1544–57.
Acknowledgements
We thank all of the study participants and other relevant parties for their cooperation in conducting the present studies. We also thank to the following investigators who made important contributions to these studies: Kensuke Usuki (NTT Medical Center Tokyo), Norifumi Tsukamoto (Gunma University Hospital), Naoki Takezako (National Hospital Organization Disaster Medical Center), Hitoshi Ohno (Tenri Hospital), Morio Matsumoto (National Hospital Organization Shibukawa Medical Center), Kyoya Kumagai (Chiba Cancer Center), Akiyoshi Miwa (Tokyo-Kita Medical Center), Chisato Mizutani (Osaka Red Cross Hospital), Yutaka Okuno (Kumamoto University Hospital), Hiroshi Ikeda (Sapporo Medical University Hospital), Takuya Komeno (National Hospital Organization Mito Medical Center), Masahiro Kizaki (Saitama Medical University), Shinichiro Okamoto (Keio University Hospital), Tadao Ishida (Japanese Red Cross Medical Center), Nobuaki Dobashi (The Jikei University Daisan Hospital), Kiyoshi Ando (Tokai University Hospital), Keita Kirito (University of Yamanashi Hospital), Michihiro Uchiyama (Suwa Red Cross Hospital), Chihiro Shimazaki (Japan Community Health care Organization Kyoto Kuramaguchi Medical Center), Kensuke Ohta (Osaka Saiseikai Nakatsu Hospital), Yasunori Ueda (Kurashiki Central Hospital), Hideki Asaoku (Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital), Masaaki Noguchi (Juntendo University Urayasu Hospital), Ichiro Hanamura (Aichi Medical University Hospital), Shinsuke Iida (Nagoya City University Hospital), Masafumi Taniwaki (University Hospital Kyoto Prefectural University of Medicine), Hirohiko Shibayama (Osaka University Hospital), Masahiro Abe (Tokushima University Hospital), Japan.
Funding
This study was funded by Fujimoto Pharmaceutical Corp.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K Suzuki served on scientific advisory boards and speakers’ bureaus for Takeda Pharmaceutical Co. Ltd., Bristol-Myers Squibb K. K., and received honoraria from Janssen Pharmaceutical K. K., Novartis Pharma K. K., Celgene K. K., Fujimoto Pharmaceutical Corp., and Takeda Pharmaceutical Co. Ltd. K. Sunami received research grants from Fujimoto Pharmaceutical Corp., Novartis Pharma K. K., GlaxoSmithKline K. K. Janssen Pharmaceutical K. K., Abbvie GK., Takeda Pharmaceutical Co. Ltd., Sanofi K. K., Bristol-Myers Squibb K. K., Ono Pharmaceutical Co. Ltd., MSD K. K., Alexion Pharma, Daiichi-Sankyo Co. Ltd., Celgene K. K., and honoraria from Takeda Pharmaceutical Co. Ltd., Bristol-Myers Squibb K. K., Ono Pharmaceutical Co. Ltd., Celgene K. K.Y. T. Takagi received consulting fees from Fujimoto Pharmaceutical Corp. H. Murakami received a research grant from Fujimoto Pharmaceutical Corp. and Celgene K. K., and served on scientific advisory boards and speakers’ bureaus for Celgene K. K., Sanofi K. K. and Ono Pharmaceutical Co. Ltd. K. Shimizu received consulting fees from Daiichi-Sankyo Co. Ltd. and Fujimoto Pharmaceutical Corp. N. Doki, K. Meguro, H. Kosugi and O. Sasaki declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Suzuki, K., Doki, N., Meguro, K. et al. Report of phase I and II trials of melphalan, prednisolone, and thalidomide triplet combination therapy versus melphalan and prednisolone doublet combination therapy in Japanese patients with newly diagnosed multiple myeloma ineligible for autologous stem cell transplantation. Int J Hematol 110, 447–457 (2019). https://doi.org/10.1007/s12185-019-02700-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02700-3