Abstract
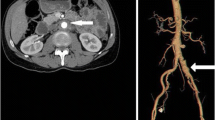
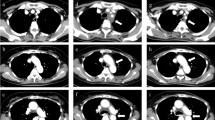
Granulocyte colony-stimulating factor (G-CSF) is commonly administered to prevent serious complications caused by chemotherapy-induced neutropenia; however, several cases of arteritis following the administration of G-CSF have been reported. Here, we report three cases of patients with non-Hodgkin lymphomas (NHLs) who developed arteritis after the administration of G-CSF, estimate the probability of adverse drug reaction caused by G-CSF with two distinct algorithms, and review the literatures. Both algorithms indicated a causal relationship between G-CSF and arteritis. In a literature review of seven reported cases, including our three patients, the time from the administration of G-CSF to the onset of arteritis ranged from 9 days to 6 months, and five patients were treated with steroids. In one of our three cases, a 62-year-old female with NHL developed arteritis twice in different courses of chemotherapy. Hydrocortisone was administered in the second event, leading to prompt relief of the manifestation and abnormal laboratory data. This finding suggests steroids may be effective for arteritis. In conclusion, although the number of reported cases is limited, there appears to be an association between arteritis and the administration of G-CSF, and steroids are an effective therapeutic option.

Similar content being viewed by others
References
Adiga GU, Elkadi D, Malik SK, Fitzpatrick JD, Madajewicz S. Abdominal aortitis after use of granulocyte colony-stimulating factor. Clin Drug Investig. 2009;29:821–5.
Darie C, Boutalba S, Fichter P, Huret J-F, Jaillot P, Deplus F, et al. Aortitis after G-CSF injections. La Rev Med interne. 2004;25:225–9.
Ito Y, Noda K, Aiba K, Yano S, Fujii T. Diffuse large B-cell lymphoma complicated with drug-induced vasculitis during administration of pegfilgrastim. Rinsho Ketsueki. 2017;58:2238–42.
Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep. 1977;61:1023–7.
Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-López AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711–6.
Ueda K, Nannya Y, Asai T, Yamamoto G, Hangaishi A, Takahashi T, et al. Efficacy and safety of modified rituximab-ESHAP therapy for relapsed/refractory B-cell lymphoma. J Chemother. 2010;22:54–7.
Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–9.
Ghimire S, Karmacharya P, Poudel DR, Bingham CO, Donato AA, Giri S, et al. Rituximab-induced serum sickness: a systematic review. Semin Arthritis Rheum. 2015;45:334–40.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
Miller EB, Grosu R, Landau Z. Isolated abdominal aortitis following administration of granulocyte colony stimulating factor (G-CSF). Clin Rheumatol. 2016;35:1655–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mineo Kurokawa: Dr. Kurokawa reports grants from Pfizer Seiyaku K.K, grants from Astellas Pharma, grants from Kyowa Hakko Kirin Co., Ltd., Grants from Takeda Pharmaceutical Company Limited., Grants from ONO PHARMACEUTICAL CO., LTD., Grants from Nippon Shinyaku Co., Ltd., Grants from Bristol-Meyer Squib, grants from Novartis Pharmaceuticals, other from Shionogi & Co., Ltd, other from Celgene K.K., outside the submitted work; Masashi Miyauchi: Dr. Miyauchi reports Grants from Kyowa Hakko Kirin Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sasaki, K., Miyauchi, M., Ogura, M. et al. Arteritis after administration of granulocyte colony-stimulating factor: a case series. Int J Hematol 110, 370–374 (2019). https://doi.org/10.1007/s12185-019-02662-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02662-6