Abstract
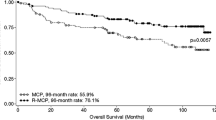
GALLIUM is a global phase III study that demonstrated a statistically significant and clinically meaningful improvement in progression-free survival (PFS) with obinutuzumab plus chemotherapy (G-chemo) versus rituximab plus chemotherapy (R-chemo) in previously untreated patients with follicular lymphoma (FL). In this single-country subgroup analysis, we explored patterns of efficacy and safety in patients enrolled in the GALLIUM study in Japan (Japanese subgroup). Patients were randomized to open-label induction treatment with G-chemo or R-chemo. Responders received maintenance monotherapy with their randomized antibody for up to 2 years. The primary endpoint was investigator-assessed PFS. Overall, 123 patients with FL were randomized in the Japanese subgroup (G-chemo, n = 65; R-chemo, n = 58). The majority of patients received cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy (82.9 vs 33.1% in the global GALLIUM FL population). PFS at 3 years was 89.9% (G-chemo) vs. 74.7% (R-chemo); hazard ratio 0.42; 95% confidence interval 0.15, 1.15; P = 0.08. Higher rates of grade 3–5 adverse events (96.9 vs. 89.7%) and serious adverse events (35.4 vs. 22.4%) were observed with G-chemo vs R-chemo, respectively. Neutropenia was frequent in the Japanese subgroup (92.3% G-chemo; 79.3% R-chemo). Overall, the results in the Japanese subgroup were consistent with those in the global GALLIUM population.



Similar content being viewed by others
References
Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32.
Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–23.
Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–92.
Salles G, Mounier N, de Guibert S, Morschhauser F, Doyen C, Rossi JF, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–31.
Rituxan injection prescribing information. 2017. http://www.info.pmda.go.jp/downfiles/ph/PDF/380101_4291407A1027_2_23.pdf. Accessed June 2018.
Izutsu K. Treatment of follicular lymphoma. J Clin Exp Hematopathol. 2014;54:31–7.
NCCN guidelines for treatment of cancer by site. B-cell lymphomas. 2017. http://www.nccn.org/. Accessed June 2018.
Igarashi T, Ogura M, Itoh K, Taniwaki M, Ando K, Kuroda Y, et al. Japanese phase II study of rituximab maintenance for untreated indolent B-cell non-Hodgkin lymphoma with high tumor burden. Int J Hematol. 2016;104:700–8.
Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–402.
Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031–42.
Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies. Adv Ther. 2017;34:324–56.
Radford J, Davies A, Cartron G, Morschhauser F, Salles G, Marcus R, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122:1137–43.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Sehn LH, Chua N, Mayer J, Dueck G, Trneny M, Bouabdallah K, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17:1081–93.
Zelenetz AD, Mobasher M, Costa LJ, Flinn I, Flowers CR, Kamnski MS, et al. Safety and efficacy of obinutuzumab (GA101) plus CHOP chemotherapy in first-line advanced diffuse large B-cell lymphoma: results from the phase 2 GATHER study. Blood. 2013;122:1820.
Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377:1331–44.
Hirayama Y, Ishitani K, Ohta S, Kurosawa M, Kondo T, Takimoto R, Kato J. Long-term survey of 443 cases of advanced-stage follicular lymphoma in Japan during the rituximab era. J Clin Oncol. 2014;32(Suppl):e19504.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: WHO Press; 2008.
Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–18.
Miyazato H, Nakatsuka S, Miyanaga I, Hanamoto H, Tatsumi Y, Matsuda M, et al. Follicular lymphoma in Osaka, Japan: histological features and chronological change. Int J Hematol. 2002;76:333–7.
Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Hematol. 2014;164:536–45.
Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65.
Brice P, Bastion Y, Lepage E, Brousse N, Haioun C, Moreau P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15:1110–7.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Rahmouni A, Luciani A, Itti E. MRI and PET in monitoring response in lymphoma. Cancer Imaging. 2005; 5(Spec No A):S106–12.
Trotman J, Luminari S, Boussetta S, Versari A, Dupuis J, Tychyj C, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1:e17–27.
Trotman J, Barrington S, Belada D, Meignan M, MacEwan R, Owen C, et al. Prognostic value of PET-CT after first-line immunochemotherapy for follicular lymphoma in the phase III GALLIUM study. Hematol Oncol. 2017;35(Suppl S2):38–40.
Pott C, Hoster E, Kehden B, Unterhalt M, Herold M, van der Jagt RH, et al. Minimal residual disease in patients with follicular lymphoma treated with obinutuzumab or rituximab as first-line induction immunochemotherapy and maintenance in the phase 3 GALLIUM study. Blood. 2016;128:613.
Esai Co Ltd. Anticancer agent Treakisym® approved in Japan for additional indication as first-line treatment for low-grade B-cell non-Hodgkin’s lymphoma and mantle cell lymphoma. 2016. http://www.eisai.com/news/news201689.html. Accessed June 2018.
Yokoyama M, Kusano Y, Takahashi A, Inoue N, Ueda K, Nishimura N, et al. Incidence and risk factors of febrile neutropenia in patients with non-Hodgkin B-cell lymphoma receiving R-CHOP in a single center in Japan. Support Care Cancer. 2017;25:3313–20.
Acknowledgements
The authors would like to thank the UK and German study groups (UK National Cancer Research Institute, German Low Grade Lymphoma Study Group and the East German Study Group Hematology and Oncology) for their scientific support of the GALLIUM study, all of the GALLIUM study investigators, their teams, and the patients for their participation. The GALLIUM trial was sponsored by F. Hoffmann-La Roche Ltd. Medical writing support for this article was provided by Lynda McEvoy, PhD (Gardiner-Caldwell Communications, Macclesfield, UK), funded by Chugai Pharmaceutical Co Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KO reports personal fees from Chugai Pharmaceutical Co Ltd, Kyowa Hakko Kirin Co Ltd, Eisai Co Ltd, Pfizer Inc and Takeda Pharmaceutical Co Ltd. KT reports Grants and personal fees from Chugai Pharmaceutical Co Ltd/Roche, Celgene, Eisai, Janssen Pharmaceuticals, Kyowa Hakko Kirin, Mundipharma, Ono Pharmaceutical and Takeda; Grants from Abbvie, GlaxoSmithKine and Servier; and personal fees from HUYA Bioscience and Zenyaku Kogyo. TK reports Grants and personal fees from Chugai Pharmaceutical Co Ltd, Ono, Gilead, MSD and Zenyaku; Grants from Takeda and Solaisia; and personal fees from Bristol, Kyowa Kirin, Eisai and Janssen. TI, KKumagai, SI, YK, IC, TC, YK, KKubo, KM and NT report no conflicts of interest. KH reports Grants from Chugai Pharmaceutical Co. KO reports Grants and personal fees from Chugai Pharmaceutical Co Ltd and Takara-Bio Inc; and personal fees from Kyowa Hakko Kirin Co Ltd, Ono Pharmaceutical Co Ltd, Bristol-Meyers Squibb, Chugai Pharmaceutical Co Ltd and Alexion Pharmaceuticals Inc. KT reports Grants from Chugai Pharmaceutical Co Ltd, Celgene, Takeda and Mundipharma; consultancy from Huya and Ono Pharma, and lecture fees from Zenyaku Kogyo, Celgene, Huya, Kyowa Hakko Kirin and Chugai Pharmaceutical Co Ltd. MT reports Grants and personal fees from Chugai/Roche, Celgene, BMS, and Janssen Pharmaceuticals; Grants from Kyowa Hakko Kirin; and personal fees from Mundipharma. TU reports personal fees from Janssen Pharmaceuticals, Mundipharma, Celgene, Teijin, Novartis, Nippon Shinyaku, Pfizer, Briston-Myers Squibb, and Meiji Seika Pharama. KI reports Grants from Kenyaku Kogyo, Mundhi, Abbvie, Solasia, Celltrion, Symbio, Astellas, Astellas Amgen, Novartis and Sanofi; Grants and personal fees from Takeda, Eisai, Chugai Pharmaceutical Co Ltd, Gilead, Janssen, Ono, Celgene, MSD, Bayer and Daiichi-Sankyo, personal fees from Kyowa Hakko Kirin; and discloses a relationship with HUYA Bioscience International. IY reports research funding and honoraria from Kyowa Hakko Kirin; research funding from Chugai; and honoraria from Celgene. FI reports Grants and personal fees from Chugai Pharmaceutical Co Ltd, Kyowa Hakko Kirin, and Takeda; Grants from Bristol-Myers-Squibb; and personal fees from Celgene. NU reports research Grants from AMED, Pfizer Co, Sysmex Co, Kyowa Hakko Kirin Co, Bristol-Myers-Squibb, Novartis, Nippon Shinyaku, Fujimoto and Celgene; payment for data monitoring committee membership from CIMIC Co, Takeda Bio Development Center, Lilly Japan, Pfizer Co, Nippon Boehringer-Ingelheim Co, Janssen Pharmaceutical Co, Zenyaku Kogyo Co, Kyowa Hakko Kirin Co, Otsuka Pharm Co, Celgene Co, SymBio Pharmaceutical Co, Huya Bioscience International, and Astellas Pharmaceutical Co; and payment for speaker’s bureau for Chugai Pharmaceutical Co Ltd and Bristol-Myers-Squibb. SI reports Grants from Chugai Pharmaceutical Co Ltd, Kyowa Hakko Kirin, Astellas, Toyama Chemicals, Teijin Pharma, Sanofi, Bayer, J-Pharma, Eli Lilly, and Daiichi Sankyo; and Grants and personal fees from Ono, Janssen, Celgene, Bristol-Myers-Squibb, Novartis, and Takeda. TM reports personal fees from Bristol-Myers-Squibb, Celgene, Esai, Janssen Pharmaceutical, Kyowa Hakko Kirin, Nippon Shinyaku, Novartis, Ono Pharmaceuticals, Otsuka Pharmaceuticals, Pfizer, Sanofi, Siemens Healthcare Diagnostics, Sumitomo Dainippon Pharma, and Taiho Pharmaceutical. EU and HK are employees of Chugai Pharmaceutical Co Ltd. HK reports stock ownership for Chugai Pharmaceutical Co Ltd. KA reports subsidies or donations from Meiji Seika Pharma Co Ltd, Takeda Pharmaceutical Co Ltd, Eizai Co Ltd, Kyowa Kirin and the Japan Blood Products Organization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ohmachi, K., Tobinai, K., Kinoshita, T. et al. Efficacy and safety of obinutuzumab in patients with previously untreated follicular lymphoma: a subgroup analysis of patients enrolled in Japan in the randomized phase III GALLIUM trial. Int J Hematol 108, 499–509 (2018). https://doi.org/10.1007/s12185-018-2497-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2497-0