Abstract
Joint bleeding and resultant arthropathy are major determinants of quality of life in haemophilia patients. We previously developed a mesenchymal stromal cell (MSC)-based treatment approach for haemophilic arthropathy in a mouse model of haemophilia A. Here, we evaluated the long-term safety of intra-articular injection of lentivirally transduced autologous MSCs in non-human primates. Autologous bone-marrow-derived MSCs transduced with a lentiviral vector expressing coagulation factor VIII (FVIII) were injected into the left knee joint of cynomolgus monkeys. We first conducted codon optimization to increase FVIII production in the cells. Lentiviral transduction of autologous MSCs resulted in a significant increase of FVIII in the culture supernatant before transplantation. We did not find any tumour generation around the knee structure at 11–16 months after injection by magnetic resonance imaging. The proviral sequence of the simian immunodeficiency virus lentiviral vector was not detected in the heart, lungs, spleen, liver, testis, or bone marrow by real-time quantitative PCR. We confirmed the long-term safety of intra-articular injection of transduced MSCs in a non-human primate. The procedure may be an attractive therapeutic approach for joint diseases in haemophilia patients.
Similar content being viewed by others
Introduction
Haemophilia is a hereditary congenital haemorrhagic disorder caused by genetic mutations in blood coagulation factor VIII (FVIII) or IX. Despite advances in treatment and comprehensive care, joint bleeding and resultant arthropathy are still considered to be the major determinants for quality of life (QOL) in haemophilia patients [1]. Regular replacement therapy with coagulation factor concentrates from early childhood is now common to prevent arthropathy in developed countries [2]. The deterioration of QOL by arthropathy may become a greater problem for the care of haemophilia patients in developing countries, where the use of factor concentrates is often limited [3]. Recent successes of adeno-associated virus (AAV) vector-mediated gene therapy indicate that gene therapy could become an alternative approach to avoid joint bleeding [4, 5]. However, increased coagulation factor levels may occasionally fail to suppress joint bleeding in cases of developed haemophilic arthropathy. This is because synovial hypertrophy and hypervascularization increase susceptibility to mechanical damage, resulting in easy bleeding [6, 7]. Therefore, new approaches that specifically treat joint bleeding and arthropathy are highly desired.
Although the pathogenesis of haemophilic arthropathy has not been fully elucidated, potential targets to treat include iron, inflammation, vascular remodeling, bone remodeling, and cartilage regeneration [8]. To effectively treat or prevent joint disease in haemophilia, we applied mesenchymal stromal cells (MSCs) as a delivery vehicle for coagulation factor, because MSCs have the potential to regulate local inflammation and tissue regeneration [9]. Indeed, the intra-articular injection of MSCs expressing FVIII previously ameliorated acute joint bleeding and resultant arthropathy in mice with haemophilia A [10]. Because of the minimally invasive procedure, this strategy may become a clinically applicable cell therapy approach to treat joint diseases in patients with haemophilia. Here, we evaluated the long-term safety of intra-articular injection of lentivirally transduced autologous MSCs in a non-human primate.
Method
cDNA construction and lentiviral vector production
cDNA for human B domain-deleted FVIII (hBDD-FVIII) was generated as described previously [11]. We synthesized two codon-optimized sequences using two different companies [Thermo Fisher Scientific (Waltham, MA) and GenScript (Piscataway, NJ)] with the aim of selecting the best sequence to express FVIII, because different algorisms may influence protein expression [12]. We designated the two CO sequences as CO-BDDhFVIII-A and CO-BDDhFVIII-B, respectively. Each cDNA was inserted into a pCDNA3 plasmid (Thermo Fisher Scientific) for transient expression in HEK293 cells. To express FVIII in MSCs, the cDNA of each hBDD-FVIII under the control of the PAI-1 promoter conjugated to the cytomegalovirus enhancer (CEP) was cloned into a self-inactivating simian immunodeficiency virus (SIV) lentiviral vector plasmid [10, 13].
The SIV lentiviral vectors were essentially generated by transfection into HEK293T cells as described previously [14]. The transduction units of the lentiviral vectors and proviral integration into genomic DNA were quantified by real-time PCR to measure the copy number of woodchuck hepatitis virus posttranscriptional regulatory element (WPRE). The oligonucleotide primer pair and FAM-labelled probe were: 5′-GCATTGCCACCACCTGTCA-3′ (sense), 5′-TCCGCCGTGGCAATAGG-3′ (anti-sense), and 5-CTTTCCGGGACTTTCG-3′ (FAM-labelled probe).
Cell culture and lentiviral transduction
Bone-marrow-derived human MSCs (hMSCs) were purchased from Lonza Walkersville, Inc. (Walkersville, MD) and maintained in accordance with the manufacturer’s recommendations. HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium/F-12 supplemented with 10% fetal bovine serum. To transduce with SIV lentiviral vectors, the cells (1 × 105/well) were seeded in a 12-well culture plate and incubated overnight. The indicated concentration of SIV lentiviral vector expressing the FVIII gene driven by the CEP promoter was added in the presence of 8 µg/ml polybrene. The medium was changed every day, and FVIII activity (FVIII:C) in the culture supernatant was measured at 96 h after transduction.
Measurement of FVIII:C
FVIII:C was measured by a one-stage clotting time assay in an automated coagulation analyser (Sysmex CA-500 analyzer; Sysmex Corp., Kobe, Japan). Pooled normal human plasma was used as a reference to measure FVIII:C.
Animals
Cynomolgus monkeys (Macaca fascicularis) were maintained at the Tsukuba Primate Research Center, National Institutes of Biomedical Innovation, Health and Nutrition (Ibaraki, Japan). All animal experiments were approved by the Institutional Animal Care and Concern Committees at Jichi Medical University and Tsukuba Primate Research Center. Animal care was performed in accordance with the committees’ guidelines.
Isolation of bone-marrow cells, culture, and transduction protocol for macaque MSCs
Bone marrow (5–10 ml) was aspirated from the posterior iliac crest of male cynomolgus monkeys anesthetized using ketamine hydrochloride (Ketalar, 10 mg/kg; intramuscularly) (Daiichi-Sankyo Inc., Tokyo, Japan). Bone marrow was diluted with PBS containing 2% human serum and DNase I (PHD buffer). Bone-marrow mononuclear cells were separated by density gradient centrifugation using Ficoll–Paque PLUS (GE Healthcare, Munich, Germany). Erythroid cells were then lysed in a hypotonic ammonium chloride buffer, followed by repeated washing with PHD buffer. The cells were resuspended in alpha minimum essential medium containing 10% fetal bovine serum and then seeded in a culture flask at a density of 5 × 105/cm2. Half of the medium volume was changed every other day, and adherent cells were defined as bone-marrow-derived MSCs. The MSCs were passaged at confluency, and passage 3 or 4 cells were transduced with the SIV lentiviral vectors at a multiplicity of infection (MOI) of 10.
Autologous transplantation of transduced MSCs into the knee joint
The cynomolgus monkeys were anesthetized using ketamine hydrochloride (10 mg/kg, intramuscularly), and hair covering the left knee joint was shaved. The transduced autologous MSCs suspended with 1 ml of medium were directly injected into the left knee joint space with a 23 G needle below the patella.
Magnetic resonance imaging (MRI)
To assess the long-term safety of transplantation of autologous MSCs transduced with a SIV lentiviral vector, we acquired the detailed anatomical structure of knee joints by MRI. The cynomolgus monkeys were anesthetized using ketamine hydrochloride (10 mg/kg, intramuscularly). Magnetic resonance images of both knee joints were then obtained by a 3T MR scanner (Magnetom Allegra; SIEMENS Healthineer, Madison, WI).
Distribution of cells transduced with SIV lentiviral vectors
After performing the MRI, cynomolgus monkeys were deeply anesthetized with a lethal dose of sodium pentobarbital. Tissue samples of the liver, heart, lungs, spleen, kidneys, bone marrow, testes, and joint capsule below the patella were obtained by necropsy. DNAs of each tissue were isolated using a DNA isolation kit (DNeasy Tissue Kit; QIAGEN Inc., Valencia, CA). Integration of the SIV lentiviral vector into genomic DNA was quantified by real-time PCR to detect the WPRE sequence as described above.
Results
We first conducted codon optimization of hBDD-FVIII to increase FVIII production in the cells. Previous reports have indicated that codon optimization significantly improves FVIII production [15, 16]. We obtained two CO hBDD-FVIII cDNA sequences and incorporated each cDNA into a pCDNA3 expression plasmid. The plasmid expressed the protein derived from the cDNA under the control of the cytomegalovirus promoter. We transduced HEK293 cells with each pCDNA3 plasmid, and then compared FVIII:C in the culture supernatants after transduction (Fig. 1a). Both CO sequences significantly increased FVIII:C in the supernatants (Fig. 1a). FVIII:C derived from the CO-BDDhFVIII-B sequence was higher than that from CO-BDDhFVIII-A.
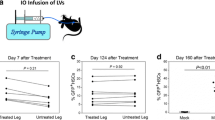
Expression of FVIII in transduced HEK 293 cells and human mesenchymal stromal cells (MSCs). a HEK293 cells were transduced with plasmids expressing control (pCDNA3), original B domain-deleted human coagulation factor VIII (BDD-FVIII), or codon-optimized BDD-FVIII (CO-BDDhFVIII-A or -B). FVIII activities (FVIII:C) in culture supernatants were measured by a one-stage clotting-time assay in an automated coagulation analyser. Values are the means ± SEM (n = 3). b HEK293 cells were transduced with a simian immunodeficiency virus lentiviral vector expressing BDD-FVIII or CO-BDDhFVIII-B at the indicated MOIs. FVIII:C in the supernatants was measured by the one-stage clotting time assay. Values are the means ± SEM (n = 3). c, d Human MSCs were transduced with the simian immunodeficiency virus lentiviral vector expressing CO-BDDhFVIII-B at the indicated MOIs. c FVIII:C in the supernatants was measured by the one-stage clotting time assay. Values are the means ± SEM (n = 3). d Proviral integrations in genomic DNA were measured by real-time quantitative PCR. Values are the means ± SEM (n = 3)
Next, we constructed an SIV lentiviral vector containing the original hBDD-FVIII or CO-BDDhFVIII-B cDNAs driven by the CEP promoter (SIV-CEP-hFVIII and SIV-CEP-COhFVIII, respectively), and then transduced HEK293 cells. The CEP promoter, in which the PAI-1 promoter is conjugated to the cytomegalovirus enhancer, appears to be stable for transduction of MSCs [10]. As shown in Fig. 1b, codon optimization significantly ameliorated the level of FVIII in the supernatant at low MOIs. The highest MOI (1) did not increase the FVIII level because of a loss of cell viability (data not shown). To determine an efficient transduction protocol for primate MSCs, we employed commercially available hMSCs. We transduced the hMSCs with several MOIs of SIV-CEP-COhFVIII, and determined FVIII:C in supernatants as well as proviral integration in the genome. FVIII:C in the supernatants and proviral integration in the genome was increased in a dose-dependent manner (Fig. 1c, d). FVIII production at an MOI of 30 did not appear to be efficient because of high proviral DNA integration. Hence, MSCs were transduced at an MOI of 10 in further experiments.
We finally transplanted autologous MSCs transduced with SIV-CEP-COhFVIII into cynomolgus monkeys. We isolated bone marrow from five male monkeys and established autologous MSCs (Table 1). Macaque MSCs were transduced with SIV-CEP-COhFVIII at an MOI of 10, and 1 × 106−7 cells were transplanted into the left knee space (Table 1). FVIII:C in the supernatant was 19–200 IU/ml before transplantation (Table 1). We further examined local tumourigenesis of transduced MSCs by MRI at 11–16 months after injection and did not find any tumour generation around the knee structure (Videos 1–5, and Fig. 2). We further examined the distribution of transduced MSCs in major organs. Proviral integration of the SIV lentiviral vector was not detected in the heart, lungs, spleen, liver, testis, and bone marrow by real-time quantitative PCR (data not shown).
Anatomical structure of knee joints observed by magnetic resonance imaging (MRI) after injection of transduced autologous mesenchymal stromal cells (MSCs). Five cynomolgus monkeys received an intra-articular injection of transduced MSCs expressing coagulation factor VIII. Magnetic resonance images of both knee joints were obtained by a 3T MR scanner (Magnetom Allegra; SIEMENS Healthineer, Madison, WI)
Discussion
Regular replacement therapy with coagulation factor concentrates from early childhood is recommended to prevent joint disease [17]. Gene therapy using AAV vectors may become an alternative approach for regular replacement therapy [4, 18]. However, developed arthropathy becomes haemorrhagic easily and deformation of the joint structure is irreversible [6]. Hence, specific treatments should be developed for progressive joint damage. We previously showed that transplantation of transduced MSCs expressing FVIII prevents blood-borne joint disease in haemophilia A mice [10]. Here, we confirmed long-term safety of transplantation of autologous MSCs transduced with an SIV lentiviral vector in a non-human primate. The confirmation of safety in large animals may lead to clinical application of the procedure.
The most important result obtained in our study is the long-term safety of lentivirally transduced MSCs in a non-human primate. We have applied a self-inactivating lentiviral vector to express coagulation factors. First generation gene therapy using retroviral vectors for the treatment of severe combined immunodeficiency patients has resulted in genotoxicity of proviral integration into genomic DNA [19, 20]. It was established that transformed cells from patients with leukaemia contained integrated vector proviruses near LMO2 (LIM domain only 2) [19, 20]. It is also known that enhancer elements in the viral long terminal repeat (LTR) are responsible for proto-oncogene activation. To reduce the genotoxicity of retroviral vectors, a self-inactivating vector, in which the promoter activity of LTR is eliminated, is mainly applied in clinical studies [21]. In addition, lentiviral vectors integrate less frequently near transcription start sites than retroviral vectors [22, 23]. Conversely, these integrative properties of lentiviral vectors maintain transgene expression even after cell division, because proviral sequences are integrated into host genomic DNA. In addition, it is unnecessary to consider the host-acquired immune system, which eliminates viral vector transduction, as in the case with AAV vectors [9]. The SIV-based vectors as used in this study are planned to apply for gene therapies of cystic fibrosis and retinitis pigmentosa, and no apparent toxicity was observed in mice and nonhuman primate models, respectively [24, 25].
MSCs are considered to be an appropriate cell source for cell-based therapy of haemophilia, because a number of clinical trials have demonstrated its safety. In 2015, 3,686 patients were treated with cellular or tissue-engineered therapies in Europe, and the most frequently used cell type was MSCs (40%) [26]. In addition, there has been clinical use of hMSCs based on the several treatment properties, including regeneration and immunomodulatory effects [26]. These properties have led to MSCs becoming an appropriate cell source to repair damaged joints. Peritoneal transplantation of bone-marrow-derived MSCs transduced with an FVIII-expressing vector ameliorated joint bleeding by local migration of the cells in a sheep model of haemophilia A [27]. It was also reported that intravenous administration of liver-derived MSCs into haemophilia A patients resulted in the distribution of MSCs in the lungs, liver, and joints [28]. However, we believe that local transplantation of MSCs may be the most realistic approach for treatment of join disease because of the safety by limiting the distribution of the transplanted cells. We previously confirmed localization of MSCs after transplantation into the joint space of mice [10]. In this study, we did not detect any migration of MSCs into other organs after long-term observation in the non-human primate. Furthermore, we increased the concentration of the cells in the joint by local injection.
A limitation of this study was that we did not assess therapeutic effects to prevent or treat arthropathy, because the employed monkeys had a normal phenotype without joint bleeding. Moreover, we did not determine the duration of maintenance of transduced MSC in the joint space. The transgene expression in transduced MSCs is preserved for at least 1 month after injection into mice [10]. However, the transplanted MSCs may not be maintained for a long time, because proviral integration was not be detected in synovial tissue (data not shown). To overcome this limitation, the transduced cells can be injected repeatedly, because arthrocentesis is a minimally invasive procedure. We have previously developed a porcine model of haemophilia A [29]. It is suitable to assess this cell-based therapy for arthropathy, because porcine haemophilia easily develops blood-borne joint damage [29]. We are now planning to examine the efficacy of the MSC-based cell therapy in the porcine model of haemophilia A.
In summary, we confirmed long-term safety of the injection of lentivirally transduced autologous MSCs into the joint space of a non-human primate. Cell therapy is an attractive approach for treating haemophilia, because local injection of transduced cells prevents systemic distribution of viral vectors, and transplantation into the joint space appears to be a minimally invasive approach.
References
Young G. New challenges in hemophilia: long-term outcomes and complications. Hematol Am Soc Hematol Educ Program. 2012;2012:362–8.
Srivastava A. Haemophilia care—beyond the treatment guidelines. Haemophilia. 2014;20(Suppl 4):4–10.
Marijke van den Berg H. Preventing bleeds by treatment: new era for haemophilia changing the paradigm. Haemophilia. 2016;22(Suppl 5):9–13.
George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377:2215–27.
Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519–30.
Nijdam A, Foppen W, van der Schouw YT, Mauser-Bunschoten EP, Schutgens RE, Fischer K. Long-term effects of joint bleeding before starting prophylaxis in severe haemophilia. Haemophilia. 2016;22:852–8.
Hanley J, McKernan A, Creagh MD, Classey S, McLaughlin P, Goddard N, et al. Guidelines for the management of acute joint bleeds and chronic synovitis in haemophilia: a United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) guideline. Haemophilia. 2017;23:511–20.
Pulles AE, Mastbergen SC, Schutgens RE, Lafeber FP, van Vulpen LF. Pathophysiology of hemophilic arthropathy and potential targets for therapy. Pharmacol Res. 2017;115:192–9.
Ohmori T, Mizukami H, Ozawa K, Sakata Y, Nishimura S. New approaches to gene and cell therapy for hemophilia. J Thromb Haemost. 2015;13(Suppl 1):S133-42.
Kashiwakura Y, Ohmori T, Mimuro J, Yasumoto A, Ishiwata A, Sakata A, et al. Intra-articular injection of mesenchymal stem cells expressing coagulation factor ameliorates hemophilic arthropathy in factor VIII-deficient mice. J Thromb Haemost. 2012;10:1802–13.
Ishiwata A, Mimuro J, Kashiwakura Y, Niimura M, Takano K, Ohmori T, et al. Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb Res. 2006;118:627–35.
Menzella HG. Comparison of two codon optimization strategies to enhance recombinant protein production in Escherichia coli. Microb Cell Fact. 2011;10:15.
Mimuro J, Muramatsu S, Hakamada Y, Mori K, Kikuchi J, Urabe M, et al. Recombinant adeno-associated virus vector-transduced vascular endothelial cells express the thrombomodulin transgene under the regulation of enhanced plasminogen activator inhibitor-1 promoter. Gene Ther. 2001;8:1690–7.
Ohmori T, Mimuro J, Takano K, Madoiwa S, Kashiwakura Y, Ishiwata A, et al. Efficient expression of a transgene in platelets using simian immunodeficiency virus-based vector harboring glycoprotein Ibalpha promoter: in vivo model for platelet-targeting gene therapy. FASEB J. 2006;20:1522–4.
McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–44.
Ward NJ, Buckley SM, Waddington SN, Vandendriessche T, Chuah MK, Nathwani AC, et al. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117:798–807.
Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding-related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2011;9:CD003429.
Arruda VR, Doshi BS, Samelson-Jones BJ. Novel approaches to hemophilia therapy: successes and challenges. Blood. 2017;130:2251–6.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9.
McCormack MP, Rabbitts TH. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2004;350:913–22.
Cavazzana M, Six E, Lagresle-Peyrou C, Andre-Schmutz I, Hacein-Bey-Abina S. Gene therapy for X-linked severe combined immunodeficiency: where do we stand? Hum Gene Ther. 2016;27:108–16.
Hematti P, Hong BK, Ferguson C, Adler R, Hanawa H, Sellers S, et al. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2004;2:e423.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–96.
Alton EW, Beekman JM, Boyd AC, Brand J, Carlon MS, Connolly MM, et al. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax. 2017;72:137–47.
Ikeda Y, Yonemitsu Y, Miyazaki M, Kohno R, Murakami Y, Murata T, et al. Acute toxicity study of a simian immunodeficiency virus-based lentiviral vector for retinal gene transfer in nonhuman primates. Hum Gene Ther. 2009;20:943–54.
Ireland H, Gay MHP, Baldomero H, De Angelis B, Baharvand H, Lowdell MW, et al. The survey on cellular and tissue-engineered therapies in Europe and neighboring Eurasian countries in 2014 and 2015. Cytotherapy. 2018;20(1):1–20.
Porada CD, Sanada C, Kuo CJ, Colletti E, Mandeville W, Hasenau J, et al. Phenotypic correction of hemophilia A in sheep by postnatal intraperitoneal transplantation of FVIII-expressing MSC. Exp Hematol. 2011;39:1124–35 e4.
Sokal EM, Lombard CA, Roelants V, Najimi M, Varma S, Sargiacomo C, et al. Biodistribution of liver-derived mesenchymal stem cells after peripheral injection in a hemophilia A patient. Transplantation. 2017;101:1845–51.
Kashiwakura Y, Mimuro J, Onishi A, Iwamoto M, Madoiwa S, Fuchimoto D, et al. Porcine model of hemophilia A. PLoS One. 2012;7:e49450.
Acknowledgements
We acknowledge Dr. Hiroaki Shibata (Tsukuba Primate Research Center) for providing the protocol to isolate monkey MSCs.
Funding
This study was supported by the Research Program on HIV/AIDS from the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP17fk0410306h0003 and JP18fk0410017h0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Ohmori received research support from Bayer, Dai-ichi Sankyo, Novo Nordisk, and CSL Behring outside of this study. M.I. and T.T. are employees of ID Pharma Inc. All other authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ohmori, T., Mizukami, H., Katakai, Y. et al. Safety of intra-articular transplantation of lentivirally transduced mesenchymal stromal cells for haemophilic arthropathy in a non-human primate. Int J Hematol 108, 239–245 (2018). https://doi.org/10.1007/s12185-018-2465-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2465-8