Abstract
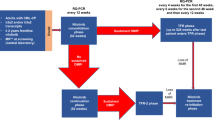
The objective of this prospective clinical trial (JALSG-STIM213, UMIN000011971) was to evaluate treatment-free remission (TFR) rates after discontinuation of imatinib in chronic myeloid leukemia (CML). CML patients who received imatinib treatment for at least 3 years and sustained deep molecular response for at least 2 years were eligible. Molecular recurrence was defined as loss of major molecular response (MMR). Of the 68 eligible patients, 38.2% were women, the median age was 55.0 years, and the median duration of imatinib treatment was 97.5 months. The 12-month TFR rate was 67.6%. Patients who lost MMR were immediately treated with imatinib again; all re-achieved MMR. Three-year treatment-free survival (TFS) was estimated as 64.6% using the Kaplan–Meier method. Undetectable molecular residual disease (UMRD) was defined as no BCR-ABL1 in > 100,000 ABL1 control genes using international scale polymerase chain reaction. UMRD at the study baseline was found to be predictive of continuation of TFR. Our findings suggest that CML patients who meet all the eligibility criteria that have commonly been used in the TFR trials are able to discontinue imatinib use safely. TFR may thus be valuable as a new goal for CML treatment in Japan.

Similar content being viewed by others
References
Garcia-Manero G, Faderl S, O’Brien S, Cortes J, Talpaz M, Kantarjian HM. Chronic myelogenous leukemia: a review and update of therapeutic strategies. Cancer. 2003;98(3):437–57.
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29.
Redaelli A, Bell C, Casagrande J, Stephens J, Botteman M, Laskin B, et al. Clinical and epidemiologic burden of chronic myelogenous leukemia. Expert Rev Anticancer Ther. 2004;4(1):85–96.
Cortes JE, Talpaz M, Kantarjian H. Chronic myelogenous leukemia: a review. Am J Med. 1996;100(5):555–70.
Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131(3):207–19.
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–27.
Kizaki M, Okamoto S, Tauchi T, et al. Current and future perspectives on the TARGET system: the registration system for Glivec established by the JSH. Int J Hematol. 2008;88(4):409–17.
Tauchi T, Kizaki M, Okamoto S, Tanaka H, Tanimoto M, Inokuchi K, et al. Seven-year follow-up of patients receiving imatinib for the treatment of newly diagnosed chronic myelogenous leukemia by the TARGET system. Leuk Res. 2011;35(5):585–90.
JSH. Clinical practice guidelines in hematology malignancy. In: Usui N, Kizaki M, Shimoda K, Takahashi N, editors. CML/MPN. Tokyo: Kanehara Shuppan, Co. Ltd.; 2013.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84.
Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–25.
Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Investig. 2011;121(1):396–409.
Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119(6):1501–10.
Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125(21):3230–5.
Gratwohl A, Brand R, Apperley J, Crawley C, Ruutu T, Corradini P, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2006;91(4):513–21.
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre stop imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–35.
Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298–305.
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–22.
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–30.
Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–35.
Lee SE, Choi SY, Song HY, Kim SH, Choi MY, Park JS, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101(6):717–23.
Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–54.
Rea D, Cayuela JM. Treatment-free remission in patients with chronic myeloid leukemia. Int J Hematol. 2017;. doi:10.1007/s12185-017-2295-0.
Matsuki E, Ono Y, Sakurai M, Kunimoto H, Ishizawa J, Shimizu T, et al. Discontinuation of imatinib in patients with CML and sustained complete molecular response (CMR) for over 2 years in the Japanese population—an interim analysis of KEIO STIM study. Blood. 2011;118(21):1608.
Takahashi N, Kyo T, Maeda Y, Sugihara T, Usuki K, Kawaguchi T, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97(6):903–6.
Mita A, Miyamura K, Hino M, Watakabe K, Takahashi K, Yoshimoto M, et al. Evaluating sensitivity of Ipsogen BCR-ABL1 Mbcr IS-MMR DX Kit for scoring molecular response. J Blood Disord Transfus. 2015;6(5):314.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
NCCN. NCCN clinical practice guidelines in oncology. Chronic myeloid leukemia; Version 2. 2017;MS18−19.
Mahon F, Richter J, Guilhot J, Hjorth-Hansen H, Almeida A, Janssen JWM, et al. Cessation of tyrosine kinase inhibitors treatment in chronic myeloid leukemia patients with deep molecular response: results of the Euro-Ski trial. Blood. 2016;128(22):787.
Ilander M, Olsson-Stromberg U, Schlums H, Guilhot J, Bruck O, Lahteenmaki H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31(5):1108–16.
Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–47.
Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435(7046):1267–70.
Roeder I, Horn M, Glauche I, Hochhaus A, Mueller MC, Loeffler M. Dynamic modeling of imatinib-treated chronic myeloid leukemia: functional insights and clinical implications. Nat Med. 2006;12(10):1181–4.
Deininger M. Hematology: curing CML with imatinib—a dream come true? Nat Rev Clin Oncol. 2011;8(3):127–8.
Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999–1003.
Mori S, Vagge E, le Coutre P, Abruzzese E, Martino B, Pungolino E, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90(10):910–4.
Hughes TP, Boquimpani CM, Takahashi N, Benyamini N, Clementino NC, Shuvaev V, et al. Treatment-free remission in patients with chronic myeloid leukemia in chronic phase according to reasons for switching from imatinib to nilotinib: subgroup analysis from ENESTop. Blood. 2016;128(22):792.
Acknowledgements
The authors would like to thank the investigators and clinical research coordinators of the sites in the JALSG study group and all of the patients who participated in this study. The Ipsogen BCR-ABL1M-BCR IS-PCR kits was provided by Sysmex Co., Ltd., and IS-PCR analysis was performed at the central laboratory of Sysmex Co., Ltd. We thank Ms. Mika Yoshimura and Mr. Yoshiro Ikeuchi at Sysmex Co., Ltd. for their collaboration. We also thank Ms. Saori Takahashi at Akita University for collecting the data and conducting central monitoring of this study. This study was supported by the Practical Research for Innovative Cancer Control program of the Japan Agency for Medical Research and Development (AMED 15ck0106189h0001, 16ck0106189h0002, 17ck0106189h0003).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Naoto Takahashi reports grants from Japan Agency for Medical Research and Development, non-financial support from Sysmex Co., Ltd., personal fees from NPO JALSG, during the conduct of the study; grants and personal fees from Novartis Pharma K.K., grants and personal fees from Otsuka Pharmaceutical Co., Ltd., grants and personal fees from Pfizer Japan Inc., personal fees from Bristol-Myers Squibb K. K., outside the submitted work. Tetsuzo Tauchi reports grants and other from Novartis Pharma K.K., grants and other from Pfizer Japan Inc., other from Bristol-Myers Squibb K. K., other from Otsuka Pharmaceutical Co., Ltd., during the conduct of the study. Koichi Miyamura reports personal fees from Novartis Pharma K.K., personal fees from Otsuka Pharmaceutical Co., Ltd., personal fees from Bristol-Myers Squibb K. K., personal fees from Pfizer Japan Inc., outside the submitted work. Kensuke Usuki reports personal fees from Novartis Pharma K.K., grants from Fujimoto Pharmaceutical, grants from Otsuka Pharmaceutical Co., Ltd., grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Kyowa Hakko Kirin Co., Ltd., grants from Daiichi Sankyo Co., Ltd., outside the submitted work. Itaru Matsumura reports personal fees from Novartis Pharma K.K., personal fees from Bristol-Myers Squibb K. K., personal fees from Pfizer Japan Inc., personal fees from Otsuka Pharmaceutical Co., Ltd., during the conduct of the study. Yosuke Minami reports grants from Kyowa Hakko Kirin Co., Ltd., grants from Novartis Pharma K.K., grants from Bristol-Myers Squibb K. K., outside the submitted work. Noriko Usui reports personal fees from CIMIC Co., Ltd., personal fees from Takeda Bio Development Center, personal fees from Lilly Japan, personal fees from Pfizer Japan Inc., grants from Pfizer Japan Inc., personal fees from Nippon Boehringer Ingelheim Co., Ltd., grants from Sysmex Co., Ltd., personal fees from Jansen Pharm Co, Ltd., personal fees from Zenyaku Kogyo Co, Ltd., personal fees from Kyowa Hakko Kirin Co., Ltd., grants from Kyowa Hakko Kirin Co., Ltd., personal fees from Otsuka Pharmaceutical Co., Ltd., personal fees from Celgene K. K., personal fees from SymBio Pharm Co, Ltd., personal fees from Huya Bioscience International, personal fees from Astellas Pharma Inc., personal fees from Chugai Pharmaceutical Co. Ltd., grants from Bristol-Myers Squibb K. K., personal fees from Bristol-Myers Squibb K. K., grants from Novartis Pharma K.K., grants from Nippon Shinyaku Co. Ltd., grants from Fujimoto Pharmaceutical, grants from Celgene K. K., outside the submitted work. Tetsuya Fukuda reports personal fees from Novartis Pharma K.K., outside the submitted work. Yoshiko Atsuta reports personal fees and other from Otsuka Pharmaceutical Co., Ltd., personal fees and other from Bristol-Myers Squibb K. K., personal fees from Mochida Pharmaceutical Co., Ltd., personal fees from Kyowa Hakko Kirin Co., Ltd., outside the submitted work. Yukio Kobayashi reports personal fees from Pfizer Japan Inc., personal fees from Otsuka Pharmaceutical Co., Ltd., personal fees from Daiichi Sankyo Co., Ltd., personal fees from CIMIC Co., Ltd., outside the submitted work. Hitoshi Kiyoi reports grants from Japan Agency for Medical Research and Development, during the conduct of the study; grants from Chugai Pharmaceutical Co. Ltd., grants and personal fees from Bristol-Myers Squibb K. K., grants from Kyowa Hakko Kirin Co. Ltd., grants from FUJIFILM Corporation, grants from Nippon Boehringer Ingelheim Co., Ltd., grants and personal fees from Astellas Pharma Inc., grants from Celgene K. K., personal fees from Daiichi Sankyo Co. Ltd., grants and personal fees from Pfizer Japan Inc., grants from Nippon Shinyaku Co. Ltd., grants from Eisai Co. Ltd., grants from Takeda Pharmaceutical Co. Ltd., grants from Ono Pharmaceutical Co. Ltd., grants from Japan Blood Products Organization, outside the submitted work. Yasushi Miyazaki reports personal fees from Sumitomo Dainippon Pharma Co., Ltd., grants and personal fees from Kyowa Hakko Kirin Co., Ltd., personal fees from Novartis Pharma K.K., personal fees from Celgene K. K., personal fees from Shinbio, grants from Chugai Pharmaceutical Co. Ltd., grants from Takeda Pharmaceutical Co. Ltd., outside the submitted work. Tomoki Naoe reports grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Fujifilm Corporation, grants from Astellas Pharma Inc., personal fees from Nippon Boehringer Ingelheim Co., Ltd., grants and personal fees from Otsuka Pharmaceutical Co., Ltd., grants from Toyoma Chemical Co., Ltd., outside the submitted work; In addition, TN has a patent Fujifilm Corporation pending. The other authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takahashi, N., Tauchi, T., Kitamura, K. et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int J Hematol 107, 185–193 (2018). https://doi.org/10.1007/s12185-017-2334-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2334-x