Abstract
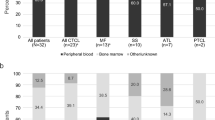
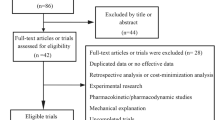
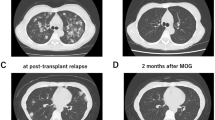
We present the interim results of a postmarketing all-case surveillance study in patients with C–C chemokine receptor 4 (CCR4)-positive, relapsed or refractory adult T-cell leukemia–lymphoma (ATL) treated with the anti-CCR4 monoclonal antibody mogamulizumab since its 2012 launch in Japan. The safety and efficacy analysis populations comprised 484 and 442 patients, respectively. The ATL subtype was acute in 58.9% and lymphoma in 34.2% of patients. All patients were scheduled to receive intravenous infusions of mogamulizumab (1.0 mg/kg) once weekly for eight weeks, alone or in combination with other modalities. Adverse drug reactions (ADRs) were reported in 74.0% of patients, of which 35.7% were serious and 6.2% were fatal. The priority survey items of infusion-related reaction, skin disorder, infection, immune disorder, and tumor lysis syndrome were reported in 29.3, 34.3, 22.1, 3.5, and 2.5% of patients, respectively. Graft-versus-host disease was reported in 25/42 patients who received mogamulizumab before allogeneic hematopoietic stem cell transplantation. The best overall response rate was 57.7% overall, 57.5% in patients treated with mogamulizumab alone, and 58.2% in patients treated with combination therapy. This surveillance indicates that mogamulizumab shows acceptable tolerability in practice; however, because of the risk of serious/fatal ADRs, patients administered mogamulizumab should be carefully monitored.

Similar content being viewed by others
References
Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92.
Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15:e517–26.
Tsukasaki K, Tobinai K. Human T-cell lymphotropic virus type I-associated adult T-cell leukemia–lymphoma: new directions in clinical research. Clin Cancer Res. 2014;20:5217–25.
Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79:428–37.
Shimoyama M. Chemotherapy of ATL. In: Takatsuki K, editor. Adult T-cell leukemia. Oxford: Oxford University Press; 1994. p. 221–37.
Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126:2570–7.
Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W Jr, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia–lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–9.
Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99:1505–11.
Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–34.
Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia–lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837–42.
Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-CC chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32:1157–63.
Ishida T, Jo T, Takemoto S, Suzushima H, Uozumi K, Yamamoto K, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol. 2015;169:672–82.
Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44 Suppl S1:1–58.
JSHCT Monograph 2008 July (in Japanese). http://www.jshct.com/guideline/pdf/2009gvhd.pdf.
Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia–lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28:1591–8.
Yonekura K, Tokunaga M, Kawakami N, Takeda K, Kanzaki T, Nakano N, et al. Cutaneous adverse reaction to mogamulizumab may indicate favourable prognosis in adult T-cell leukaemia-lymphoma. Acta Derm Venereol. doi:10.2340/00015555-2421 (Epub ahead of print).
Azukizawa H, Sano S, Kosaka H, Sumikawa Y, Itami S. Prevention of toxic epidermal necrolysis by regulatory T cells. Eur J Immunol. 2005;35:1722–30.
Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182:8071–9.
Shiratori S, Ohigashi H, Ito S, Kudo K, Adachi M, Minamimoto T, et al. Late onset toxic epidermal necrolysis induced by mogamulizumab, an anti-CC chemokine receptor 4 antibody for the treatment of adult T-cell leukaemia/lymphoma. Hematol Oncol. 2017;35:138–40.
Ishida T, Ito A, Sato F, Kusumoto S, Iida S, Inagaki H, et al. Stevens–Johnson syndrome associated with mogamulizumab treatment of adult T-cell leukemia/lymphoma. Cancer Sci. 2013;104:647–50.
Honda T, Hishizawa M, Kataoka TR, Ohmori K, Takaori-Kondo A, Miyachi Y, et al. Stevens-Johnson Syndrome associated with mogamulizumab-induced deficiency of regulatory T cells in an adult T-cell leukaemia patient. Acta Derm Venereol. 2015;95:606–7.
Tanba K, Uoshima N, Uchiyama H, Kawata E, Isa R, Yamaguchi J, et al. Toxic epidermal necrolysis in adult T cell leukemia/lymphoma treated with mogamulizumab. Ann Hematol. 2016;95:661–2.
Tay MR, Lim ST, Tao M, Quek RH, Tay K, Tan TT. Cytomegalovirus infection and end-organ disease in Asian patients with lymphoma receiving chemotherapy. Leuk Lymphoma. 2014;55:182–7.
Ishii Y, Itabashi M, Numata A, Yamamoto W, Motohashi K, Hagihara M, et al. Cytomegalovirus pneumonia after anti-CC-chemokine receptor 4 monoclonal antibody (mogamulizumab) therapy in an angioimmunoblastic T-cell lymphoma patient. Intern Med. 2016;55:673–5.
Ohyama Y, Kumode T, Eguchi G, Yamaguchi T, Maeda Y. Induction of molecular remission by using anti-CC-chemokine receptor 4 (anti-CCR4) antibodies for adult T cell leukemia: a risk of opportunistic infection after treatment with anti-CCR4 antibodies. Ann Hematol. 2014;93:169–71.
Tamaki K, Kinjo T, Aoyama H, Tomoyose T, Nakachi S, Hanashiro T, et al. Fatal pneumonia and viremia due to human parainfluenza virus type 1 in a patient with adult T-cell leukemia–lymphoma treated with mogamulizumab. J Infect Chemother. 2015;21:820–3.
Totani H, Kusumoto S, Ishida T, Masuda A, Yoshida T, Ito A, et al. Reactivation of hepatitis B virus (HBV) infection in adult T-cell leukemia–lymphoma patients with resolved HBV infection following systemic chemotherapy. Int J Hematol. 2015;101:398–404.
Ifuku H, Kusumoto S, Tanaka Y, Totani H, Ishida T, Okada M, et al. Fatal reactivation of hepatitis B virus infection in a patient with adult T-cell leukemia–lymphoma receiving the anti-CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res. 2015;45:1363–7.
Morichika K, Tomoyose T, Hanashiro T, Shimabukuro N, Tamaki K, Tedokon I, et al. Recurrence of psoriasis vulgaris accompanied by treatment with C–C chemokine receptor type 4 (CCR4) antibody (mogamulizumab) therapies in a patient with adult T cell leukemia/ lymphoma: insight into autoinflammatory diseases. Intern Med. 2016;55:1345–9.
Fuji S, Inoue Y, Utsunomiya A, Moriuchi Y, Uchimaru K, Choi I, et al. Pretransplantation anti-CCR4 antibody mogamulizumab against adult T-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34:3426–33.
Sugio T, Kato K, Aoki T, Ohta T, Saito N, Yoshida S, et al. Mogamulizumab treatment prior to allogeneic hematopoietic stem cell transplantation induces severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2016;22:1608–14.
Haji S, Kiyasu J, Choi I, Suehiro Y, Toyoda K, Tsuda M, et al. Administration of an anti-CC chemokine receptor 4 monoclonal antibody, mogamulizumab, before allogeneic bone marrow transplantation for adult T-cell leukemia/lymphoma. Bone Marrow Transplant. 2016;51:432–4.
Inoue Y, Fuji S, Tanosaki R, Fukuda T. Pretransplant mogamulizumab against ATLL might increase the risk of acute GVHD and non-relapse mortality. Bone Marrow Transplant. 2016;51:725–7.
Ito Y, Miyamoto T, Chong Y, Aoki T, Kato K, Akashi K, et al. Successful treatment with anti-CC chemokine receptor 4 MoAb of relapsed adult T-cell leukemia/lymphoma after umbilical cord blood transplantation. Bone Marrow Transplant. 2013;48:998–9.
Yonekura K, Kanzaki T, Gunshin K, Kawakami N, Takatsuka Y, Nakano N, et al. Effect of anti-CCR4 monoclonal antibody (mogamulizumab) on adult T-cell leukemia–lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol. 2014;41:239–44.
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–94.
Acknowledgements
The authors wish to thank Denise Daley, MD, and Marion Barnett of Edanz Group Japan K.K. for providing medical writing support during the development of this manuscript, which was funded by Kyowa Hakko Kirin Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KI reports grants and personal fees from Kyowa Hakko Kirin, Chugai Pharmaceutical, Takeda Pharmaceutical, Novartis, Eisai, and Taiho Pharmaceutical; personal fees from Bristol-Myers Squibb, Celgene, Janssen Pharmaceutical, and Pfizer; and grants from Yakult Pharmaceutical, MSD, and Japan Blood Products Organization, outside the submitted work. SY, KK, and YT are employees and stockholders of Kyowa Hakko Kirin. MI and TT are employees of Kyowa Hakko Kirin. KT reports grants and personal fees from Eisai, Takeda, Janssen, and Mundipharma; personal fees from Zenyaku Kogyo, and HUYA Bioscience International; and grants from Chugai Pharma, Kyowa Hakko Kirin, Ono Pharmaceutical Celgene, GlaxoSmithKline, SERVIER, and Abbvie, outside the submitted work.
Funding
This study was funded by Kyowa Hakko Kirin Co., Ltd.
About this article
Cite this article
Ishitsuka, K., Yurimoto, S., Kawamura, K. et al. Safety and efficacy of mogamulizumab in patients with adult T-cell leukemia–lymphoma in Japan: interim results of postmarketing all-case surveillance. Int J Hematol 106, 522–532 (2017). https://doi.org/10.1007/s12185-017-2270-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2270-9