Abstract
Objective
Positron emission tomography (PET) is a non-invasive technique measuring quantification of physiological and biochemical processes in the living organism. However, there are many considerations including anesthesia and fasting to acquire small animal imaging. We aimed to evaluate the effects of anesthesia and fasting of rats in dopamine transporter (DAT) imaging acquisition.
Methods
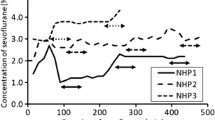
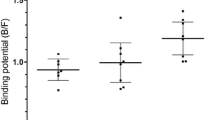
Male Sprague Dawley (SD) rats aged 7 weeks and weighing 180–260 g were used in this study. Rats were randomly divided by 4 groups. Group A was kept under anesthesia for 40 min and fasted over 12 h. Group B was only fasted over 12 h. Group C was only kept under anesthesia for 40 min. Group D was neither kept under anesthesia nor fasted over 12 h. PET scans were started at 40 min after 18F-FP-CIT injection and obtained for 20 min. Volumes-of-interest for striatum and extrastriatal area were used for 18F-FP-CIT PET analysis. Cerebellum was considered as a reference region. Specific binding ratio (SBR) was calculated as follows: [(uptake of target-uptake of cerebellum)]/(uptake of cerebellum).
Results
SBR without fasting and anesthesia (group D) was significantly lower than those of other groups (vs group A, p = 0.0004; vs group B, p = 0.0377; vs group C, p = 0.0134). However, SBRs of extrastriatal area (p = 0.5120) were not affected by fasting and anesthesia.
Conclusions
In conclusion, the SBR of striatum was increased after anesthesia by isoflurane and fasting. When designing an experiment using DAT imaging, the effects of isoflurane and fasting should be considered.




Similar content being viewed by others
Change history
06 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12149-023-01842-z
References
Tremoleda JL, Kerton A, Gsell W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012;2(1):44.
Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J. 2008;49(1):17–26.
Momosaki S, Hatano K, Kawasumi Y, Kato T, Hosoi R, Kobayashi K, et al. Rat-PET study without anesthesia: anesthetics modify the dopamine D1 receptor binding in rat brain. Synapse. 2004;54(4):207–13.
Mantz J, Varlet C, Lecharny JB, Henzel D, Lenot P, Desmonts JM. Effects of volatile anesthetics, thiopental, and ketamine on spontaneous and depolarization-evoked dopamine release from striatal synaptosomes in the rat. Anesthesiology. 1994;80(2):352–63.
Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–78.
Roseberry AG. Acute fasting increases somatodendritic dopamine release in the ventral tegmental area. J Neurophysiol. 2015;114(2):1072–82.
Papp EA, Leergaard TB, Calabrese E, Johnson GA, Bjaalie JG. Waxholm Space atlas of the Sprague Dawley rat brain. Neuroimage. 2014;97:374–86.
Schiffer WK, Mirrione MM, Biegon A, Alexoff DL, Patel V, Dewey SL. Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J Neurosci Methods. 2006;155(2):272–84.
Khan KS, Hayes I, Buggy DJ. Pharmacology of anaesthetic agents II: inhalation anaesthetic agents. Contin Edu Anaesth Critic Care Pain. 2013;14(3):106–11.
Friedman JA, Khurana VG, Anderson RE, Meyer FB. Cerebral Blood Flow: Physiology and Measurement Techniques. In: Moore AJ, Newell DW, editors. Neurosurgery: Principles and Practice. London: Springer; 2005. p. 301–314.
Nakagawara J. Functional neuroimagings “Overview”. In: Cho BK, Tominaga T, editors. Moyamoya disease update. Tokyo: Springer; 2010.
Waelbers T, Peremans K, Gielen I, Vermeire S, Polis I. Brain perfusion, part 2: anesthesia and brain perfusion in small animals. Vlaams Diergeneeskundig Tijdschrift. 2010;79(3):179–88.
Van Aken H, Fitch W, Graham DI, Brussel T, Themann H. Cardiovascular and cerebrovascular effects of isoflurane-induced hypotension in the baboon. Anesth Analg. 1986;65(6):565–74.
McPherson RW, Traystman RJ. Effects of isoflurane on cerebral autoregulation in dogs. Anesthesiology. 1988;69(4):493–9.
Reinstrup P, Ryding E, Algotsson L, Berntman L, Uski T. Regional cerebral blood flow (SPECT) during anaesthesia with isoflurane and nitrous oxide in humans. Br J Anaesth. 1997;78(4):407–11.
Li CX, Patel S, Wang DJ, Zhang X. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging. 2014. https://doi.org/10.1016/j.mri.2014.04.019.
Ori C, Dam M, Pizzolato G, Battistin L, Giron G. Effects of isoflurane anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1986;65(2):152–6.
Lenz C, Rebel A, van Ackern K, Kuschinsky W, Waschke KF. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 1998;89(6):1480–8.
Spangler-Bickell MG, de Laat B, Fulton R, Bormans G, Nuyts J. The effect of isoflurane on (18)F-FDG uptake in the rat brain: a fully conscious dynamic PET study using motion compensation. EJNMMI Res. 2016;6(1):86.
Matsumura A, Mizokawa S, Tanaka M, Wada Y, Nozaki S, Nakamura F, et al. Assessment of microPET performance in analyzing the rat brain under different types of anesthesia: comparison between quantitative data obtained with microPET and ex vivo autoradiography. Neuroimage. 2003;20(4):2040–50.
Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N, et al. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res. 1999;849(1–2):85–96.
Votaw J, Byas-Smith M, Hua J, Voll R, Martarello L, Levey AI, et al. Interaction of isoflurane with the dopamine transporter. Anesthesiology. 2003;98(2):404–11.
Byas-Smith MG, Li J, Szlam F, Eaton DC, Votaw JR, Denson DD. Isoflurane induces dopamine transporter trafficking into the cell cytoplasm. Synapse. 2004;53(2):68–73.
Ishida A, Nakajima W, Takada G. Short-term fasting alters neonatal rat striatal dopamine levels and serotonin metabolism: an in vivo microdialysis study. Brain Res Dev Brain Res. 1997;104(1–2):131–6.
Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68(1):11–20.
Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, et al. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81(4):859–69.
Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543.
Kameyama M, Ishibash K, Wagatsuma K, Toyohara J, Ishii K. A pitfall of white matter reference regions used in [18F] florbetapir PET: a consideration of kinetics. Ann Nucl Med. 2019;33(11):848–54.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, S., Kim, K., Pak, K. et al. Effects of animal handling on striatal DAT availability in rats. Ann Nucl Med 34, 496–501 (2020). https://doi.org/10.1007/s12149-020-01476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01476-5