Abstract
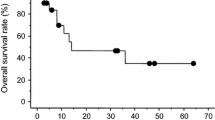
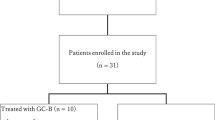
The objective of this study was to analyze the clinical outcomes of TIP (paclitaxel, ifosfamide and cisplatin) incorporated into induction chemotherapy for patients with metastatic germ cell tumor (GCT) characterized by unfavorable clinical features. This study included 37 patients, who were categorized into intermediate- or poor-risk GCT according to the International Germ Cell Consensus Classification (IGCCC). All 37 patients received two cycles of bleomycin, etoposide and cisplatin (BEP) followed by several cycles of TIP. Following treatment with TIP, 25 patients achieved the normalization of serum tumor markers. In addition, surgical resection of the residual tumors following TIP was performed in 17 patients who were pathologically diagnosed with no viable cancer cells. At a median follow-up of 36 months, 31 patients were alive, including 27 with no evidence of disease, whereas the remaining six died of disease progression. The 5-year disease-free survival (DFS) and overall survival (OS) rates in these 37 patients were 72.9 and 85.3 %, respectively. Despite the lack of a significant predictor of OS, univariate analysis identified the presence of a choriocarcinoma element and IGCCC as significant predictors of DFS, of which only the presence of a choriocarcinoma element appeared to be independently associated with DFS. In this series, treatment-related death did not occur, although 27 patients had at least one adverse event corresponding to grade 3≤. Collectively, it would be of worth to pursue the significance of the early incorporation of TIP into induction chemotherapy for patients with intermediate- or poor-risk metastatic GCT in the future.

Similar content being viewed by others
References
Purdue MP, Devesa SS, Sigurdson AJ, et al. International patterns and trends in testis cancer incidence. Int J Cancer. 2005;115:822–7.
Nakamura T, Miki T. Recent strategy for the management of advanced testicular cancer. Int J Urol. 2010;17:148–57.
Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–84.
Motzer RJ, Nichols CJ, Margolin KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. 2007;25:247–56.
Kaye SB, Mead GM, Fossa S, et al. Intensive induction-sequential chemotherapy with BOP/VIP-B compared with treatment with BEP/EP for poor prognosis metastatic nonseminomatous germ cell tumor: a randomized Medical Research Council/European Organization for Research and Treatment of Cancer study. J Clin Oncol. 1998;16:692–701.
Farhat F, Culine S, Théodore C, et al. Cisplatin and ifosfamide with either vinblastine or etoposide as salvage therapy for refractory or relapsing germ cell tumor patients: the Institut Gustave Roussy experience. Cancer. 1996;77:1193–7.
Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:1287–93.
Bokemeyer C, Beyer J, Metzner B, et al. Phase II study of paclitaxel in patients with relapsed or cisplatin-refractory testicular cancer. Ann Oncol. 1996;7:31–4.
Motzer RJ, Sheinfeld J, Mazumdar M, et al. Paclitaxel, ifosfamide, and cisplatin second-line therapy for patients with relapsed testicular germ cell cancer. J Clin Oncol. 2000;18:2413–8.
Mead GM, Cullen MH, Huddart R, et al. A phase II trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second-line (post-BEP) salvage chemotherapy for patients with metastatic germ cell cancer: a medical research council trial. Br J Cancer. 2005;93:178–84.
Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549–55.
International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603.
Kumano M, Miyake H, Hara I, et al. First-line high-dose chemotherapy combined with peripheral blood stem cell transplantation for patients with advanced extragonadal germ cell tumors. Int J Urol. 2007;14:336–8.
Stang A, Jansen L, Trabert B, et al. Survival after a diagnosis of testicular germ cell cancers in Germany and the United States, 2002–2006: a high resolution study by histology and age. Cancer Epidemiol. 2013;37:492–7.
Habuchi T, Kamoto T, Hara I, et al. Factors that influence the results of salvage surgery in patients with chemorefractory germ cell carcinomas with elevated tumor markers. Cancer. 2003;98:1635–42.
Mosharafa AA, Foster RS, Leibovich BC, et al. Histology in mixed germ cell tumors. Is there a favorite pairing? J Urol. 2004;171:1471–3.
Cheville JC. Classification and pathology of testicular germ cell and sex cord-stromal tumors. Urol Clin N Am. 1999;26:595–609.
Terakawa T, Miyake H, Muramaki M, et al. Salvage chemotherapy with methotrexate, etoposide and actinomycin D in men with metastatic nonseminomatous germ cell tumors with a choriocarcinoma component: a preliminary report. Int J Urol. 2010;17:881–5.
Mazumdar M, Bajorin D, Bacik J, et al. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J Clin Oncol. 2001;19:2534–41.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishikawa, M., Miyake, H., Muramaki, M. et al. Efficacy and tolerability of TIP (paclitaxel, ifosfamide and cisplatin) incorporated into induction chemotherapy for patients with intermediate- or poor-risk metastatic germ cell tumors. Med Oncol 31, 296 (2014). https://doi.org/10.1007/s12032-014-0296-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0296-x