Abstract
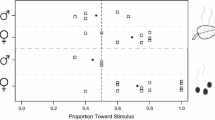
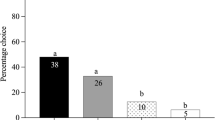
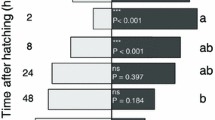
A native parasitoid, Apanteles polychrosidis, shifted hosts to exploit the invasive leaf miner, Caloptilia fraxinella, on horticultural ash, Fraxinus spp. in Edmonton, AB, Canada. A. polychrosidis has the potential to control populations of the invasive leaf miner, and parasitism rates are studied on two host plants, black ash, F. nigra, and green ash, F. pennsylvanica. Parasitism by A. polychrosidis of C. fraxinella differs on the two ash species. Parasitism is independent of leaf miner density on black ash, but is negatively density dependent on green ash. On green but not black ash, the host plant appears to mediate the numerical response of the parasitoid. Parasitoids are less effective at high host densities on green ash which may be because foraging behavior is not enhanced by leaf miner activity on green ash. Thirteen volatile organic chemicals (VOCs) released by green ash are detected by the antennae of A. polychrosidis, and eleven are identified here. The potential for host location mediated by VOCs is examined with olfactometer studies. Parasitoid females are differentially attracted to volatile cues of each ash species. Undamaged and mechanically damaged green ash leaflets attract female parasitoids, but in black ash, only leaflets mined by host larvae are attractive to parasitoids in olfactometer tests. These results suggest that A. polychrosidis uses host location cues induced by feeding damage on black ash but not on green ash. This differential attraction to VOCs from each ash species may mediate the differential parasitism observed in field studies.




Similar content being viewed by others
References
Barbosa P, Segarra AE, Gross P, Caldas A, Ahlstrom K, Carlson RW, Ferguson DC, Grissell EE, Hodges RW, Marsh PM, Poole RW, Schauff ME, Shaw SR, Whitfield JB, Woodley NE (2001) Differential parasitism of macrolepidopteran herbivores on two deciduous tree species. Ecology 82:698–704
Benrey B, Denno RF (1997) The slow-growth-high mortality hypothesis: a test using the cabbage butterfly. Ecology 78:987–999
Benrey B, Denno RF, Kaiser L (1997) The influence of plant species on attraction and host acceptance in Cotesia glomerata (Hymenoptera: Braconidae). J Insect Behav 10:619–630
Bruce TJA, Matthes MC, Chamberlain K, Woodcock CM, Mohib A, Webster B, Smart LE, Birkett MA, Pickett JA, Napier JA (2008) cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc Natl Acad Sci 105:4553–4558
Chong JH, Oetting RD (2006) Functional response and progeny production of Madeira mealy bug parasitoid, Anagyrus sp. Nov. nr. sinope: the effects of host and parasitoid densities. Biocontrol 39:320–328
Connor EF, Beck MW (1993) Density-related mortality in Cameraria hamadryadella (Lepidoptera: Gracillariidae) at epidemic and endemic densities. Oikos 66:515–525
Connor EF, Cargain MJ (1994) Density-related foraging behavior in Closterocerus tricinctus, a parasitoid of the leaf-mining moth, Cameraria hamadryadella. Ecol Entomol 19:327–334
Connor EC, Rott AS, Samietz J, Dorn S (2007) The role of the plant in attracting parasitoids: response to progressive mechanical wounding. Entomol Exp Appl 125:145–155
Cossentine J, Jensen L, Deglow E, Bennet A, Goulet H, Huber J, O’hara J (2004) The parasitoid complex affecting Choristoneura rosaceana and Pandemis limitata populations in organically managed apple orchards. Biocontrol 49:359–372
Cossentine JE, Deglow EK, Jensen LBM, Goulet H (2005) Biological assessment of Macrocentrus linearis and Apanteles polychrosidis (Hymenoptera: Braconidae) as parasitoids of the obliquebanded leafroller, Choristoneura rosaceana (Lepidoptera: Tortricidae). BioControl Sci Techn 15:711–720
Crawley MJ (2007) The R book. Wiley, West Sussex
Degen T, Bakalovic N, Bergvinson D, Turlings TCJ (2012) Differential performance and parasitism of caterpillars on maize inbred lines with distinctly different herbivore- induced volatile emissions. PLoS ONE. doi:10.1371/journal.pone.0047589
DeLury NC, Gries R, Gries G, Judd GJR, Khaskin G (1999) Moth scale-derived kairomones used by egg-larval parasitoid Ascogaster quadridentata to locate eggs of its host, Cydia pomonella. J Chem Ecol 25:2419–2431
DeMoraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore infested plants selectively attract parasitoids. Nature 393:570–573
Evenden ML (2009) Biology of Caloptilia fraxinella (Lepidoptera: Gracillariidae) on ornamental green ash, Fraxinus pennsylvanica (Oleaceae). Can Entomol 141:31–39
Evenden ML, Armitage G, Lau R (2007) Effects of nutrition and methoprene treatment on reproductive diapause in Caloptilia fraxinella (Lepidoptera: Gracillariidae). Phys Entomol 32:275–282
Farkas TE, Singer MS (2014) Can caterpillar density or host-plant quality explain host-plant related parasitism of a generalist forest caterpillar assemblage? Oecologia 173:971–983
Farrar JL (1998) Trees in Canada [CD-ROM]. Canadian Forest Service, Ottawa
Fernandez-Arhex V, Corley JC (2003) The functional response of parasitoids and its implications for biological control. Biocontrol Sci Techn 13:403–413
Fox J <jfox@mcmaster.ca > with contributions from Andronic L, Ash M, Boye T, Calza S, Chang, Grosjean AP, Heiberger R, Kerns JG, Lancelot R, Lesnoff M, Ligges U, Messad S, Maechler M, Muenchen R, Murdoch D, Neuwirth E, Putler D, Ripley B, Ristic M, Wolf P (2011) Rcmdr: R Commander. R package version 1.7. http://CRAN.Rproject.org/package=Rcmdr
Francis F, Vandermoten S, Verheggen F, Lognay G, Haubruge E (2005) Is (E)-beta farnesene the only volatile terpenoid in aphids? J Appl Entomol 129:6–12
Geervliet JBF, Ariens S, Dicke M, Vet LEM (1998) Long-distance assessment of patch profitability through volatile infochemicals by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae). Biol Control 11:113–121
Geervliet JBF, Verdel MSW, Schaub J, Snellen H, Dicke M, Vet LEM (2000) Coexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C. rubecula in the Netherlands: predicting field performance from laboratory data. Oecologia 124:55–63
Girling RD, Stewart-Jones A, Dherbecourt J, Staley JT, Wright DJ, Poppy GM (2011) Parasitoids select plants more heavily infested with their caterpillar hosts: a new approach to aid interpretation of plant headspace volatiles. Proc R Soc Biol Sci 278:2646–2653
Gries R, Khaskin G, Gries G, Bennett RG, King GGS, Morewood P, Slessor KN, Morewood WD (2002) (Z, Z)-4,7-Tridecadien-(S)-2-yl acetate: sex pheromone of douglas-fir cone gall midge, Contarinia oregonensis. J Chem Ecol 28:2283–2297
Hassell MP (2000) The spatial and temporal dynamics of host parasitoid interactions. Oxford University Press, London, Oxford Series in Ecology and Evolution
Hayashi N, Komae H (1980) Components of the ant secretions. Biochem Syst Ecol 8:293–295
Hoballah MEF, Tamò C, Turlings TCJ (2002) Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: is quality or quantity important? J Chem Ecol 28:951–968
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
James DG (2003) Synthetic herbivore-induced plant volatiles as field attractants for beneficial insects. Environ Entomol 32:977–982
James DG (2005) Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. J Chem Ecol 31:481–495
James DG, Price TS (2004) Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J Chem Ecol 30:1613–1628
Legaspi JC, French V, Zuñinga AG, Legaspi BC Jr (2001) Population dynamics of the citrus leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae), and its natural enemies in Texas and Mexico. Bio Cont 21:84–90
Lengwiler U, Turlings TCJ, Dorn S (1994) Chemically mediated host searching behavior in a parasitoid of Phyllonorycter blancardella F. (Lepidoptera, Gracillariidae) on apple. Norw J Ag Sci 16:401
Leopold EJ (1986, 1990) Selective hydroboration of a 1,3,7-triene: Homogeraniol. Org Synth 64:164 (1986); Coll. Vol. 7, 258 (1990)
Lessells CM (1985) Parasitoid foraging: should parasitism be density dependent? J Anim Ecol 54:27–41
Lill JT, Marquis RJ, Ricklefs RE (2002) Host plants influence parasitism of forest caterpillars. Nature 417:170–173
Liu S, Jiang LH (2003) Differential parasitism of Plutella xylostella (Lepidoptera: Plutellidae) larvae by the parasitoid Cotesia plutellae (Hymenoptera: Braconidae) on two host plant species. B Entomol Res 93:65–72
Lou Y, Hua X, Turlings TCJ, Cheng J, Chen X, Ye G (2006) Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. J Chem Ecol 32:2375–2387
Mahmoudi M, Sahragard A, Sendi JJ (2010) Foraging efficiency of Lysiphlebus fabarum Marshall (Hymenoptera: Aphiidae) parasitizing the black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae), under laboratory conditions. J Asia Pacific Entomol 13:111–116
Miller PF (1973) The biology of some Phyllonorycter species (Lepidoptera: Gracillariidae) mining leaves of oak and beech. J Nat Hist 7:391–409
Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on Lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol 137:1160–1168
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88:628–667
Poelman EH, Oduor AMO, Broekgaarden C, Hordijk CA, Jansen JJ, Van Loon JJA, Van Dam NM, Dicke M (2009) Field parasitism rates of caterpillars on Brassica oleracea plants are reliably predicted by differential attraction of Cotesia parasitoids. Funct Ecol 23:951–962
Pohl GR, Saunders C, Barr WB, Wartenbe MD, Fownes SL (2004) Caloptilia fraxinella (Lepidoptera: Gracillariidae), a new pest of ash (Oleaceae: Fraxinus spp.) on the Canadian prairies. Can Entomol 136:733–736
Pureswaran DS, Poland TM (2009) Host selection and feeding preference of Agrilus planipennis (Coleoptera: Buprestidae) on ash (Fraxinus spp.). Environ Entomol 38:757–765
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.Rproject.org/
Roßbach A, Löhr B, Vidal S (2005) Generalism versus specialism: responses of Diadegma mollipla (Holmgren) and Diadegma semiclausum (Hellen), to the host shift of the diamondback moth (Plutella xylostella L.) to peas. J Insect Behav 18:491–503
Roland J (1986) Parasitism of winter moth in British Colombia during build-up of its parasitoid Cyzenis albicans: attack rate on oak vs. apple. J Anim Ecol 55:215–234
Romeis J, Shanower TG, Zebitz CPW (1997) Volatile plant infochemicals mediate plant preference of Trichogramma chilonis. J Chem Ecol 23:2455–2465
Rose USR, Alborn HT, Makranczy G, Lewis WJ, Tumlinson JH (1997) Host recognition by the specialist endoparasitoid Microplitis croceipes (Hymenoptera: Braconidae): role of host and plant related volatiles. J Insect Behav 10:313–330
Rose USR, Lewis WJ, Tumlinson JH (1998) Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J Chem Ecol 24:303–319
Sasso R, Iodice L, Woodcock CM, Pickett JA, Guerrieri E (2009) Electrophysiological and behavioral responses of Aphidius ervi (Hymenoptera: Braconidae) to tomato plant volatiles. Chemoecology 19:195–201
Sato H, Okabayashi Y, Kamijo K (2002) Structure and function of parasitoid assemblages associated with Phyllonorycter leaf miners (Lepidoptera: Gracillariidae) on deciduous oaks in Japan. Environ Entomol 31:1052–1061
Schnee C, Kollner TG, Hald M, Turlings TCJ, Gershenzon J, Degenhardt J (2006) The products of a single maize sesquisterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Nat Acad Sci USA 103:1129–1134
Schwartzberg EG, Böröczky K, Tumlinson JH (2011) Pea aphids, Acyrthosiphon pisum, suppress induced plant volatiles in broad bean, Vicia faba. J Chem Ecol 37:1055–1062
Seaman AJ, Nyrop JP, Dennehy TJ (1990) Egg and larval parasitism of the grape berry moth (Lepidoptera: Tortricidae) in three grape habitats in New York. Environ Entomol 19:764–770
Shiojiri K, Ozawa R, Matsui K, Kishimoto K, Kugimiya S, Takabayashi J (2006) Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J Chem Ecol 32:969–979
Shiojiri K, Ozawa R, Kugimiya S, Uefune M, van Wijk M, Sabelis MW, Takabayashi J (2010) Herbivore-specific, density-dependent induction of plant volatiles: honest or “cry wolf” signals? PLoS ONE 5:e12161. doi:10.1371/journal.pone.0012161
Simpson M, Gurr GM, Simmons AT, Wratten SD, James DG, Leeson G, Nicol HI (2011) Insect attraction to synthetic herbivore-induced plant volatile-treated field crops. Ag For Entomol 13:45–57
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–3
Stiling PD (1987) The frequency of density dependence in insect host-parasitoid systems. Ecology 68:844–856
Turlings TCJ, Tumlinson JH, Eller FJ, Lewis WJ (1991) Larval damaged plants: source of volatile synomones that guide the parasitoid Cotesia marginiventris to the microhabitat of its hosts. Entomol Exp Appl 58:75–82
Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92:4169–4174
Van den Dool H, Kratz PD (1963) A generalization of the retention index system including temperature programmed gas liquid partition chromatography. J Chromatogra 2:463–471
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tri-trophic context. Ann Rev Entomol 37:141–172
Vinson SB (1976) Host selection by insect parasitoids. Ann Rev Entomol 21:109–133
Visser JH (1986) Host odour perception in phytophagous insects. Ann Rev Entomol 31:121–144
Wei J, Kang L (2006) Electrophysiological and behavioral responses of a parasitic wasp to plant volatiles induced by two leaf miner species. Chem Senses 31:467–477
Wei J, Wang L, Zhu J, Zhang S, Nandi OI (2007) Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 2:e852. doi:10.1371/journal.pone.0000852
Wist TJ, Evenden ML (2013) Parasitoid complex and bionomics of Apanteles polychrosidis (Hymenoptera: braconidae) on the ash leaf-cone roller (Lepidoptera: Gracillariidae). Can Entomol 145:416–429
Wist TJ, Gries R, Evenden ML (2014) The use of plant volatiles for host location by an ash (Fraxinus) specialist, Caloptilia fraxinella. Chemoecology 24:229–242
Acknowledgments
We thank Ilona Houston, Andrew Perri, and Jocelyn Walker for assistance with field work. We are indebted to José Fernandez and Henri Goulet (AAFC Ottawa) for identification of A. polychrosidis. Thanks are due to Wilbert Ronald and Jeffries Nurseries of Portage la Prairie, Manitoba, for donating the ash seedlings. Thanks are due to Nadir Erbilgin for improving an earlier version of this manuscript. We are indebted to the National Science and Engineering Research Council (NSERC), Alberta Innovates, and the Alberta Government for scholarships to TJW that funded this research and the Alberta Crop Industry Development Fund (ACIDF) and Landscape Alberta Nursery Trades Association (LANTA) for their generous grants (MLE).
Contributions of authors
TW designed, executed, and analyzed most experiments under the supervision of ME. RG performed the GC-EAD and MS analysis of the headspace volatiles of ash. TW wrote the manuscript, and ME revised each draft.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Heikki Hokkanen.
Rights and permissions
About this article
Cite this article
Wist, T.J., Greis, R. & Evenden, M.L. Differential parasitism by a generalist parasitoid is mediated by volatile organic chemicals of the herbivore’s host. Arthropod-Plant Interactions 9, 515–527 (2015). https://doi.org/10.1007/s11829-015-9393-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-015-9393-9