Abstract
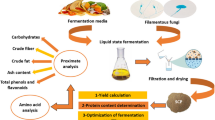
The study goal was to determine the optimal fungal culture to reduce glucosinolates (GLS), fiber, and residual sugars while increasing the protein content and nutritional value of canola meal. Solid-state incubation conditions were used to enhance filamentous growth of the fungi. Flask trials were performed using 50 % moisture content hexane-extracted (HE) or cold-pressed (CP) canola meal with incubation for 168 h at 30 °C. Incubation on HE canola meal Trichoderma reesei (NRRL-3653) achieved the greatest increase in protein content (23 %), while having the lowest residual levels of sugar (8 % w/w) and GLS (0.4 μM/g). Incubation on CP canola meal Trichoderma reesei (NRRL-3653), A. pullulans (NRRL-58522), and A. pullulans (NRRL-Y-2311-1) resulted in the greatest improvement in protein content (22.9, 16.9 and 15.4 %, respectively), while reducing total GLS content from 60.6 to 1.0, 3.2 and 10.7 μM/g, respectively. HE and CP canola meal GLS levels were reduced to 65.5 and 50.7 % by thermal treatments while solid-state microbial conversion further reduced GLS up to 99 and 98 %, respectively. Fiber levels increased due to the concentration effect of removing oligosaccharides and GLS.


Similar content being viewed by others
Abbreviations
- ADF:
-
Acid detergent fiber
- CP:
-
Cold-pressed
- dw:
-
Dry weight
- GLS:
-
Glucosinolate
- GYE:
-
Glucose yeast extract
- HE:
-
Hexane-extracted
- LC–MS:
-
Liquid chromatography–mass spectrometry
- NDF:
-
Neutral detergent fiber
- PDA:
-
Potato dextrose agar
- q-tof:
-
Quadrupole time-of-flight
- rpm:
-
Revolutions per minute
- RS:
-
Residual sugar
- RP-HPLC:
-
Reverse phase high performance liquid chromatography
- SLR:
-
Solid loading rate
References
Crop Production (2014) U.S. Canola Association. http://www.uscanola.com/crop-production. Accessed 23 Oct 2014
Newkirk R (2009a) Canola meal feed industry guide, 4 edn, pp 1–48. https://cigi.ca/wp-content/uploads/2011/12/2009-Canola_Guide
Lomascolo A, Uzan-Boukhris E, Sigoillot JC, Fine F (2012) Rapeseed and sunflower meal: a review on biotechnology status and challenges. Appl Microbiol Biotechnol 95:1105–1114
Tripathi MK, Mishra AS (2007) Glucosinolates in animal nutrition: a review. Anim Feed Sci Technol 132:1–27
Rask L, Andreasson E, Ekborn B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol 42:93–113
Kliebenstein DJ, Kroymann J, Mitchell-Olds T (2005) The glucosinolate-myrosinase system in a ecological and evolutionary context. Curr Opin Plant Biol 8:264–271
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Burel C, Boujard T, Kaushik SJ, Boeuf G, Mol KA, Van der Geyten S, Darras VM, Kuhn ER, Pradet-Balade B, Querat B, Quinsac A, Krouti M, Ribaillier D (2001) Effects of rapeseed meal-glucosinolates on thyroid metabolism and feed utilization in rainbow trout. Gen Comp Endocrinol 124:343–358
Bell JM (1993) Factors affecting the nutritional value of canola meal: a review. Can J Anim Sci 73:679–697
Newkirk RW, Maenz DD, Classen HL (2006) Oilseed Processing. US Patent 7,090,887 B2
Newkirk RW, Maenz DD, Classen HL (2009b) Defatted canola flake, protein, and phytate; isoelectric precipitation and/or ultrafiltration. US Patent 7,629,014 B2
Seiboth B, Ivanova C, Seidl-Seiboth V (2011) Trichoderma reesei: a fungal enzyme producer for cellulosic biofuels. In: dos Santos Bernardes MA (ed) Biofuel production—recent developments and prospects. 1. INTECH, Rijeka, pp 309–340
Wiebe MG (2002) Myco-protein from Fusarium venenatum: a well-established product for human consumption. Appl Microbiol Biotechnol 58:421–427
Ratledge C (2013) In: McNeil B, Archer D, Giavasis I, Harvey L (eds) Microbial production of food ingredients, enzymes and nutraceuticals. Woodhead Publishing Limited, Sawston, Cambridge, England
Olempska-Beer ZS, Merker RI, Ditto MD, DiNovi MJ (2006) Food-processing enzymes from recombinant microorganisms—a review. Regul Toxicol Pharmacol 45:144–158
Prajapati VD, Jani GK, Khanda SM (2013) Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 95:540–549
Ogunremi OR, Agrawal R, Sanni AI (2015) Development of cereal-based functional food using cereal-mix substrate fermented with probiotic strain—Pichia kudravzevii OG32. Food Sci Nutr 3(6):486–494
Couto SR, Sanromán MÁ (2006) Application of solid-state fermentation to food industry—a review. J Food Eng 76:291–302
Pandey A, Soccol CR, Mitchell D (2000) New developments in solid state fermentation: I-bioprocesses and products. Process Biochem 35:1153–1169
Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V, Szakacs G, Pandey A (2004) Coconut oil cake—a potential raw material for the production of alpha-amylase. Bioresour Technol 93:169–174
Singhania RR, Patel AK, Soccol CR, Pandey A (2009) Recent advances in solid-state fermentation. Biochem Eng J 44:13–18
Smits JP, Knol W, Bol J (1993) Glucosinolate degradation by Aspergillus clavatus and Fusarium oxysporum in liquid and solid state fermentation. Appl Microbiol Biotechnol 38:696–701
Holker U, Hofer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
O’Donnell K (2000) Molecular phylogeny of the Nectria haematococca–Fusarium solani species complex. Mycologia 92:919–938
Berhow MA, Polat U, Glinski JA, Glensk M, Vaughn SF, Isbell T, Ayala-Diaz I, Marek L, Gardner C (2013) Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Ind Crops Prod 43:119–125
Betz JM, Fox W (1994) In: Huang M, Osawa T, Ho C, Rosen TR (ed) Food phytochemicals for cancer prevention I: fruits and vegetables. ACS symposium series. American Chemical Society, Washington, DC
Saha BC (2004) Production, purification and properties of endoglucanase from a newly isolated strain of Mucor circinelloides. Process Biochem 39:1871–1876
Bhargav S, Panda BP, Ali M, Javed S (2008) Solid-state fermentation–an overview. Chem Biochem Eng Q 22:49–70
Bogar B, Szakacs G, Linden JC, Pandey A, Tengerdy RP (2003) Optimization of phytase production by solid substrate fermentation. J Ind Microbiol Biotechnol 30:183–189
Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Bock SA, Fox SL, Gibbons WR (1997) Development of a low cost, industrially suitable medium for production of acetic acid from glucose by Clostridium thermoaceticum. Biotechnol Appl Biochem 25:117–125
Haron MJ, Jahangirian H, Ismail MHS, Rafiee-Moghaddam R, Rezayi M, Shameli K, Gharayebi Y, Abdollahi Y, Peyda M, Mahdavi B (2013) Antifungal properties of phenyl fatty hydroxamic acids and their copper complexes synthesized based on canola and palm kernel oils. Asian J Chem 25:4183–4188
Spragg J, Mailer R (2007) Canola meal value chain quality improvement. Australian Oilseeds Federation and Pork CRC
Salihu A, Alam MZ, AbdulKarim MI, Salleh HM (2012) Lipase production: an insight in the utilization of renewable agricultural residues. Resour Conserv Recycl 58:36–44
Toivari M, Vehkomaki ML, Nygard Y, Penttila M, Ruohonen L, Wiebe MG (2013) Low pH d-xylonate production with Pichia kudriavzevii. Bioresour Technol 133:555–562
Hutkins RW (2006) Microbiology and technology of fermented foods, 1st edn. Blackwell Publishing, Ames, p 441
Li C, Yang Z, Zhang RH, Zhang D, Chen S, Ma L (2013) Effect of pH on cellulase production and morphology of Trichoderma reesei and the application in cellulosic material hydrolysis. J Biotechnol 168:470–477
Brown M, Gibbons WR (2014) High value protein replacers for aquaculture feed and industrial products from defatted soybean white flakes and meal. In: USB domestic opportunities meeting, Feb 4, Little Rock
Bertolin TE, Schmidell W, Maiorano AE, Casara J, Costa JAV (2003) Influence of carbon, nitrogen and phosphorous sources on glucoamylase production by Aspergillus awamori in solid state fermentation. Z Naturforsch C: J Biosci 58:708–712
Membrillo I, Sanchez C, Meneses M, Favela E, Loera O (2008) Effect of substrate particle size and additional nitrogen source on production of lignocellulolytic enzymes by Pleurotus ostreatus strains. Bioresour Technol 99:7842–7847
Rakariyatham N, Sakorn P (2002) Biodegradation of glucosinolates in brown mustard seed meal (Brassica juncea) by Aspergillus sp. NR-4201 in liquid and solid state cultures. Biodegradation 13:395–399
Vig AP, Walia A (2001) Beneficial effects of Rhizopus oligosporus fermentation on reduction of glucosinolates, fiber and phytic acid in rapeseed (Brassica napus) meal. Bioresour Technol 78:309–312
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases—production, applications and challenges. J Sci Ind Res 64:832–844
Karki B, Muthukumarappan K, Gibbons WR (2013) Optimization of extrusion processing conditions on enzymatic hydrolysis and fermentation of distillers’ low oil wet cake. In: North Central Branch American Society of Microbiology, 73rd annual meeting, 11–12 Oct, Brookings, SD
Maesoomi SM, Ghorbani GR, Alikhani M, Nikkhah A (2006) Canola meal as a substitute for cottonseed meal in diet of midlactation Holsteins. J Dairy Sci 89:1673–1677
Murray DD (2004) Canola protein concentrate as a feed ingredient for salmonid fish. In: Cruz Suarez LE, Ricque Marie D, Nieto Lopez MG, Villarreal D, Scholz U, Gonzalez M (ed). Advances en Nutricion Acuicola VIII. Memorias del VII Simposium Internacional de Nutricion Acuicola, 16–19 Nov, Hermosillo, Sonora, Mexico
Chen Y, Guo J, Li F, Liu M, Zhang X, Guo X, Xiao D (2014) Production of pullulan from xylose and hemicellulose hydrolysate by Aureobasidium pullulans AY82 with pH control and DL-dithiothreitol addition. Biotechnol Bioprocess Eng 19:282–288
Gordon C, Thomas S, Griffen A, Robson GD, Trinci A, Wiebe MG (2000) Combined use of growth rate correlated and growth rate independent promoters for recombinant glucoamylase production in Fusarium venenatum. FEMS Microbiol Lett 194:229–234
Acknowledgments
This work was performed in the Alfred Dairy Science Hall and was supported by the South Dakota Oilseed Initiative and a grant from the South Dakota Oilseed Commission. We would like to thank Kerry O’Donnell and the Bacterial Foodborne Pathogens and Mycology Research Unit of the United States Department of Agriculture for identifying the M. circincelloides and P. kudriavzevii strains used in this research. We would also like to thank Ray Holloway, Vanessa Voelker, and Sandra M. Duval for their technical assistance in the GLS analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
It is declared that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. We wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome. The manuscript has been read and approved by all authors.
Additional information
Mark Berhow: Mention of trade names or commercial products in this paper is solely for the purpose of providing specific information and does not imply endorsement by the U.S. Department of Agriculture (USDA). The USDA is an equal opportunity provider and employer.
About this article
Cite this article
Croat, J.R., Berhow, M., Karki, B. et al. Conversion of Canola Meal into a High-Protein Feed Additive via Solid-State Fungal Incubation Process. J Am Oil Chem Soc 93, 499–507 (2016). https://doi.org/10.1007/s11746-016-2796-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2796-7