Abstract
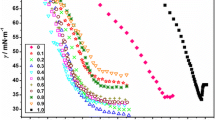
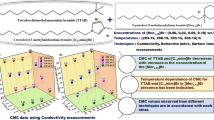
The self-aggregation of sodium dodecylsulphate (SDS), an anionic surfactant, in aqueous solutions of tetraalkylammonium bromide salts (R4NBr, where R = propyl, butyl and pentyl) was determined at various temperatures in the range 288.15–318.15 K. The critical micelle concentration (CMC) determined from conductivity data was used to study the thermodynamics of the surfactant. The presence of bromide salts was found to affect the micellization of SDS in accordance with the hydrophobicity of the tetraalkylammonium cations, thus the CMC values follow the order no additive > Pr4NBr > Bu4NBr > Pen4NBr. The results from conventional conductivity methods were combined with those of spectroscopic techniques like fluorescence and UV–Vis studies.







Similar content being viewed by others
References
Starks CM. Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. J Am Chem Soc. 1971;93:195–9.
Halpren M, Sasson Y, Robinovitz M. Hydroxide ion initiated reactions under phase transfer catalysis conditions–IV: effect of catalyst. Tetrahedron. 1982;38:3183–7.
Lygo B, Andrews BI, Crosby J, Peterson JA. Asymmetric alkylation of glycine imines using in situ generated phase-transfer catalysts. Tetrahedron Lett. 2002;43:8015–8.
Barney R, Carroll J IV, Delaet D. Surfactant studies of quaternary ammonium compounds: critical surfactant concentration. J Surfact Deterg. 2006;9:137–40.
Taylor RB, Toasaksiri S, Raid RG. Determination of antibacterial quaternary ammonium compounds in lozenges by capillary electrophoresis. J Chromat A. 1998;798:335–43.
Lopaz JR, Videa M. Study of the ion transfer of quaternary ammonium ions by SWV. J Mex Chem Soc. 2012;56:417–25.
Deakyne CA, Meot-Ner M. Unconventional ionic hydrogen bonds. 2. NH+···Π-complexes of onium ions with olefins and benzene derivatives. J Am Chem Soc. 1985;107:474–9.
Ghammani S, Sajabi SA. Tetrabutylammonium fluorochromate(VI) (TBAFC): a mild and efficient reagent for oxidation of organic substrates. J Serb Chem Soc. 2005;70:1243–8.
Vashkov VI. Antimicrobial agents and desinfection methods against infectious diseases. Moscow: Meditsina; 1997.
McBain AJ, Ledder RG, Moore LE, Catrenich CE, Gilbert P. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2004;70:3449–56.
Patel J, Varade D, Bahadur P. Effect of tetraalkylammonium bromides on the micellar behaviour of ionic and non-ionic surfactants. IJC. 2004;43A:715–21.
Mata J, Varade D, Ghosh G, Bahadur P. Effect of tetrabutylammonium bromide on the micelles of sodium dodecyl sulphate. Colloid Surf A. 2004;245:69–73.
Patist A, Huibers PDT, Deneka B, Shah DO. Effect of tetraalkylammonium chlorides on foaming properties of sodium dodecyl sulfate solutions. Langmuir. 1998;14:4471–4.
Matsouka K, Chiba N, Yoshimura T, Takeuchi E. Effect of double quaternary ammonium groups on micelle formation of partially fluorinated surfactant. J Colloid Interf Sci. 2011;356:624–9.
Yoshimura T, Chiba N, Matsouka K. Supra-long chain surfactants with double or triple quaternary ammonium headgroups. J Colloid Interf Sci. 2012;374:157–63.
Chauhan S, Kaur M, Kumar K, Chauhan MS. Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J Chem Thermodyn. 2014;78:175–81.
Chauhan S, Kumar K, Singh K, Jyoti J. Volumetric, compressibility, and surface tension studies on micellization behavior of SDS in aqueous medium: effect of sugars. J Surfact Deterg. 2014;17:169–75.
Das D, Ismail K. Aggregation and adsorption properties of sodium dodecyl sulfate in water–acetamide mixtures. J Colloid Interf Sci. 2008;327:198–203.
Kang KH, Kim HU, Lim KH. Effect of temperature on critical micelle concentration and thermodynamic potentials of micellization of anionic ammonium dodecyl sulfate and cationic octadecyl trimethyl ammonium chloride. Colloid Surf A. 2001;189:113–21.
Mata J, Varade D, Bahadur P. Aggregation behavior of quaternary salt based cationic surfactants. Thermochim Acta. 2005;428:147–55.
Mehta SK, Bhasin KK, Chauhan R, Dham S. Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloid Surf A. 2005;255:153–7.
Noudeh GD, Housaindokht M, Bazzaz BSF. The effect of temperature on thermodynamic parameters of micellization of some surfactants. J Appl Sci. 2007;7:47–52.
Prasad M, Chakraborty I, Rakshit AK, Moulik SP. Critical evaluation of micellization behavior of nonionic surfactant MEGA 10 in comparison with ionic surfactant tetradecyltriphenylphosphonium bromide studied by microcalorimetric method in aqueous medium. J Phys Chem B. 2006;110:9815–21.
Chauhan S, Sharma K. Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution: a conductometric study. J Chem Thermodyn. 2014;71:205–11.
Behera K, Pandey S. Modulating properties of aqueous sodium dodecyl sulphate by adding hydrophobic ionic liquid. J Colloid Interf Sci. 2007;316:803–14.
Ali A, Ansari NH. Studies on the effect of amino acids/peptide on micellization of SDS at different temperatures. J Surfact Deterg. 2010;13:441–9.
Javadian S, Nasiri F, Heydari A, Yousefi A, Shahir AA. Modifying effect of imidazolium-based ionic liquids on surface activity and self-assembled nanostructures of sodium dodecyl sulfate. J Phys Chem B. 2014;118:4140–50.
Das C, Das B. Effect of tetraalkylammonium salts on the micellar behavior of lithium dodecyl sulfate: a conductometric and tensiometric study. J Mol Liq. 2008;137:152–8.
Sinha S, Bahadur P. Effect of organic counter-ions on the surface activity, micellar formation and dye solubilization behaviour of cationic surfactants. IJC. 2002;41A:914–20.
Kabir-ud-din, Kumar S, Parveen N. The clouding phenomenon for anionic sodium dodecyl sulfate + quaternary bromides in polar nonaqueous-water-mixed solvents. J Surfact Deterg. 2008;11:335–41.
Yan Z, Bai X, Liu R, Wu S, Wang J. Effect of dipeptides on the micellization and thermodynamic parameters of sodium dodecyl sulfonate: conductometric and fluoimetric studies. J Mol Liq. 2013;177:78–84.
Wagle VB, Kothari PS, Gaikar VG. Effect of temperature on aggregation behavior of aqueous solutions of sodium cumene sulfonate. J Mol Liq. 2007;133:68–76.
Lumry R, Rajender S. Enthalpy–entropy compensation phenomena in water solutions of proteins and small molecules: a ubiquitous property of water. Biopolymers. 1970;9:1125–7.
Perez AG, Castillo JLD, Czapkiewicz J, Rodriguez JR. Micellization of decyl- and dodecyldimethylbenzylammonium bromides at various temperatures in aqueous solutions. Colloid Polym Sci. 2002;280:503–8.
Chen LJ, Lin SY, Huang CC. Effect of hydrophobic chain length of surfactants on enthalpy-entropy compensation of micellization. J Phys Chem B. 1998;102:4350–6.
Tanhaei B, Saghatoleslami N, Chenar MP, Ayati A, Hesampour M, Manttari M. Experimental study of CMC evaluation in single and mixed surfactant systems, using the UV–Vis spectroscopic method. J Surfact Deterg. 2013;16:357–62.
Kumar K, Chauhan S. Surface tension and UV-visible investigations of aggregation and adsorption behaviour of NaC and NaDC in water-amino acid mixtures. Fluid Phase Equilib. 2015;394:165–74.
Khan AM, Shah SS. A UV-visible study of partitioning of pyrene in an anionic surfactant sodium dodecyl sulfate. J Dispers Sci Techn. 2008;29:1401–7.
Hait SK, Moulik SP, Palepu R. Refined method of assessment of parameters of micellization of surfactants and percolation of W/O microemulsions. Langmuir. 2002;18:2471–6.
Aguiar J, Carpena P, Molina-Bolívar JA, Carnero Ruiz C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interf Sci. 2003;258:116–22.
Ali A, Tariq M, Patel R, Ittoo FA. Interaction of glycine with cationic, anionic, and nonionic surfactants at different temperatures: a volumetric, viscometric, refractive index, conductometric, and fluorescence probe study. Colloid Polym Sci. 2008;286:183–90.
Pal A, Chaudhary S. Effect of hydrophilic ionic liquid on aggregation behaviour of aqueous solutions of sodium dodecylsulfate (SDS). Fluid Phase Equilib. 2013;352:42–6.
Yoshimura T, Nagata Y, Esumi K. Interactions of quaternary salt-type gemini surfactants with sodium poly(styrene sulfonate). J Colloid Interf Sci. 2004;275:618–22.
Zhang X, Jackson JK, Burt HM. Determination of surfactant critical micelle concentration by a novel fluorescence depolarization technique. J Biochem Biophys Methods. 1996;31:145–50.
Ruiz CC. Micelle formation and microenvironmental properties of sodium dodecyl sulfate in aqueous urea solutions. Colloid Surf A. 1999;147:349–57.
Dominguez A, Fernandez A, Gonzalez N, Iglesias E, Montenegro L. Determination of critical micelle concentration of some surfactants by three techniques. J Chem Educ. 1997;74:1227–31.
Acknowledgements
S. Chauhan and Maninder Kaur are highly thankful to UGC, New Delhi for financial assistance under the project (F. No. 42-249/2013/SR) and award of Senior Research Fellowship (No. F.17-40/2008(SA-1) dated 31.07.2014), respectively. Financial support from UGC-SAP (DRS-I) (No. F.540/3/DRS/2010 (SAP-1)) to the Department of Chemistry, HPU is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Chauhan, S., Kaur, M. Modulation of Aggregation Behaviour of Anionic Surfactant in the Presence of Aqueous Quaternary Ammonium Salts. J Surfact Deterg 20, 599–607 (2017). https://doi.org/10.1007/s11743-017-1949-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-1949-5