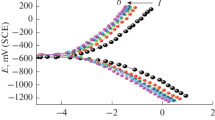
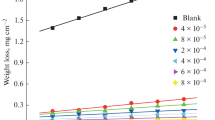
Abstract
Nonionic Schiff base surfactants were synthesized by chemical modification of tannic acid. The surface activities of the synthesized surfactants were determined using surface tension, interfacial tension, and emulsification properties. Thermodynamic parameters of adsorption and micellization of these surfactants showed their tendency towards the two processes with greater predominance of adsorption over micellization. Electrochemical polarization and impedance measurements showed that the surfactants exhibited good tendency towards inhibiting the dissolution of carbon steel in acidic medium. The inhibition efficiencies depend on the chemical structure and concentration of the compounds.





Similar content being viewed by others
References
Mahdavian M, Tehrani-Begha AR, Holmberg K (2011) Comparison of cationic gemini surfactant and the corresponding monomeric surfactant for corrosion protection of mild steel in hydrochloric acid. J Surfactants Deterg 14:605–613
Ajmal M, Rawat J, Quraishi MA (1999) Thioamidines as novel class of corrosion inhibitors. Br Corros J 34:220–226
Tawfik SM, Sayed A, Aiad I (2012) Corrosion inhibition by some cationic surfactants in oil fields. J Surfactants Deterg 15:577–585
Negm NA, Zaki MF, Said MM, Morsy SM (2011) Inhibitory action of biodegradable modified vanillin on the corrosion of carbon steel in 1 M HCl. Corros Sci 53:4233–4240
Pillai K, Narayan R (1978) Inhibition of corrosion of iron in acids by thiourea and derivatives. J Electrochem Soc 125:1393–1402
Negm NA, El Farargy AF, Al Sabagh AM, Abdelrahman NR (2011) New Schiff base cationic surfactants: surface and thermodynamic properties and applicability in bacterial growth and metal corrosion prevention. J Surfactants Deterg 14:505–514
Kendig MW (2004) US Patent application 20040175587A1
Yıldırım A, Ozturk S, Cetin M (2013) Novel amide-based cationic surfactants as efficient corrosion inhibitors for carbon steel in HCl and H2SO4 media. J Surfactants Deterg 16:13–23
Negm NA, Zaki MF (2008) Corrosion inhibition efficiency of nonionic Schiff base amphiphiles of p-aminobenzoic acid for aluminum in 4 N HCl. Colloids Surf A 322:97–102
El-Etre AY (2001) Inhibition of acid corrosion of aluminum using vanillin. Corros Sci 43:1031–1039
Shahidi M, Sasaei E, Ganjehkaviri M, Gholamhosseinzadeh MR (2012) Investigation of vanillin as a green corrosion inhibitor for stainless steel using electrochemical techniques. J Phys Theory Chem 9:149–161
Rosliza R, Noraaini A, Wan Nik WB (2010) Study on the effect of vanillin on the corrosion inhibition of aluminum alloy. J Appl Electrochem 40:833–840
Kusmierek E, Chrzescijanska E (2015) Tannic acid as corrosion inhibitor for metals and alloys. Mater Corros 66:169–174
Antonijevic MM, Petrovic MB (2008) Copper corrosion inhibitors. Int J Electrochem Sci 3:1–28
Umoren SM, Obot IB, Ebenso EE, Okafor PC, Ogobe O, Oguzie EE (2006) Gum Arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti Corros Methods Mater 53:277–282
El-Etre AY, Abdallah M, El-Tantawy ZE (2005) Corrosion inhibition of some metals using Lawsonia extract. Corros Sci 47:385–395
Aiad IA, Negm NA (2009) Some Schiff base surfactants as steel corrosion inhibitors. J Surfactants Deterg 12:313–319
Negm NA, Aiad IA (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surfactants Deterg 10:87–92
Quraishi MA, Khan MAW, Ajmal M, Muralidharan S, Iyer SV (1997) Influence of heterocyclic anils on corrosion inhibition and hydrogen permeation through mild steel in acid chloride environments. Corrosion 53:475–481
Negm NA, Morsy SMI, Said MM (2005) Corrosion inhibition of some novel hydrazone derivatives. J Surfactants Deterg 8:95–98
Sayed GH, Ghuiba GM, Abdou MI, Badr EA, Tawfik SM, Negm NA (2011) Synthesis, surface and thermodynamic parameters of some biodegradable nonionic surfactants derived from tannic acid. Colloids Surf A 393(2011):96–104
Negm NA, Kandile NG, Aiad IA, Mohammad MA (2011) New eco-friendly environmentally cationic surfactants: synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl. Colloids Surf A 391:224–233
Negm NA, Mohamed AS (2008) Synthesis, characterization and biological activity of sugar-based gemini cationic amphiphiles. J Surfactants Deterg 11:215–221
Negm NA (2007) Solubilization, surface active and thermodynamic parameters of gemini amphiphiles bearing nonionic hydrophilic spacer. J Surfactants Deterg 8:71–80
Negm NA (2005) Zwitterionic surfactants: synthesis, surface and thermodynamic properties of N-ethyl alkanoate-N-ethanol-N-methyl-N-carboxymethyl betaine Egypt. J Petrol 14:1–8
John H (1992) Surfactant aggregation, vol 4. Blackie, London
Negm NA, Mohamed AS (2004) Surface and thermodynamic properties of diquaternary bola-form amphiphiles containing aromatic spacer. J Surfactants Deterg 7:23–30
Zana R (1996) Progress in synthesis in gemini surfactants. Curr Opin Colloid Interface Sci 1:566–572
Rosen MJ (1989) Surface and interfacial phenomena, 2nd edn. Wiley, New York, p 151
Negm NA, El Farargy AFM, Mohammed DE, Mohamad HN (2015) Environmentally friendly nonionic surfactants derived from tannic acid: synthesis, characterization and surface activity. J Surfactants Deterg 15:433–443
Sowada R (1994) The effect of electrolytes on the critical micelle concentration on ionic surfactants. Tenside Surfactants Deterg 31:4–9
Bentiss F, Bouanis M, Mernari B, Traisnel M, Vezin H, Lagrenee M (2006) Understanding the adsorption of 4H-1,2,4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl Surf Sci 253:3696–3703
Behpour M, Ghoreishi SM, Salavati-Niasari M, Ebrahimi B (2008) Evaluating two new synthesized S–N Schiff bases on the corrosion of copper in 15 % hydrochloric acid. Mater Chem Phys 107:153–157
Negm NA, Al Sabagh AM, Migahed MA, Abdel Bary HM, El Din HM (2010) Effectiveness of some diquaternary ammonium surfactants as corrosion inhibitors for carbon steel in 0.5 M HCl solution. Corros Sci 52:2122–2132
Ajmal M, Miden AS, Quraishi MA (1994) 2-Hydrazino-6-methyl-benzothiazole as an effective inhibitor for the corrosion of mild steel in acidic solutions. Corros Sci 36:79–84
Asefi D, Arami M, Sarabi AA, Mahmoodi NM (2009) The chain length influence of cationic surfactant and role of nonionic cosurfactants on controlling the corrosion rate of steel in acidic media. Corros Sci 51:1817–1821
El Achouri M, Infante MR, Izquierdo F, Kertit S, Gouttaya HM, Nciri B (2009) Synthesis of some cationic gemini surfactants and their inhibitive effect on iron corrosion in hydrochloric acid medium. Corros Sci 43:19–35
Negm NA, Elkholy NA, Zahran MK, Tawfik SM (2010) Corrosion inhibition efficiency and surface activity of surface active benzothiazol-3-ium cationic schiff base derivatives in hydrochloric acid. Corros Sci 52:3523–3536
Negm NA, Ghuiba EM, Mahmoud SA, Tawfik SM (2011) Biocidal and anti-corrosive activities of benzoimidazol-3-ium cationic Schiff base surfactants. Eng Life Sci 11:496–510
Negm NA, Elkholy NA, Tawfik SM (2011) Corrosion inhibition of carbon steel by some quaternary surface active isoxazol-2-ium cationic Schiff bases in hydrochloric acid solution. Corros Sci 53:3566–3575
Negm NA, Badr EA, Aiad IA, Zaki MF, Said MM (2012) Investigation the inhibitory action of novel diquaternary diSchiff bases on the acid dissolution of carbon steel in 1 M hydrochloric acid solution. Corros Sci 65:77–86
Negm NA, Kandile NG, Mohammed MA, Bedr EA (2012) Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for dissolution of carbon steel in 1 M HCl. Corros Sci 65:94–103
Ozcan M, Dehri J, Erbil M (2004) Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl Surf Sci 236:155–164
Chauhan LR, Gunasekaran G (2007) Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros Sci 49:1143–1161
Kalman E, Varhegyi B, Felhosi I, Karman FH, Shaban A (1994) Corrosion Inhibition by 1-hydroxy-ethane-1,1-diphosphonic acid: an electrochemical impedance spectroscopy study. J Elecrochem Soc 141:3357–3360
Elkadi L, Marnari B, Traisnel M, Bentiss F, Lagrenee M (2000) The inhibition action of 3,6-bis(2-methoxyphenyl)-1,2-dihydro-1,2,4,5-tetrazine on the corrosion of mild steel in acidic media. Corros Sci 42:703–719
Quraishi MA, Rawat J (2001) Influence of iodide ions on inhibitive performance of tetraphenyl-dithia-octaaza-cyclotetradeca-hexaene (PTAT) during pickling of mild steel in hot sulfuric acid. Mater Chem Phys 70:95–99
Oguzie EE, Li Y, Wang FH (2007) Effect of 2-amino-3-mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid. Electrochim Acta 53:909–914
El-Mahdy GA, Atta AM, Al-Lohedan HA (2013) Water soluble nonionic Rosin surfactants as corrosion inhibitor of carbon steel in 1 M HCl. Int J Electrochem Sci 8:5052–5066
Shalaby MN, Osman MM (2002) Application of some commercial nonionic surfactants in the field of corrosion inhibition. Mater Corros 53:827–832
Abdallah M, El-Etre AY (2013) Corrosion inhibition of nickel in sulfuric acid using tween surfactants. Portug Electrochim Acta 21:315–326
Sobhia M, Abdallah M, Hfaeza E (2013) Some polysorbate compounds as corrosion inhibitors for carbon steel in hydrochloric acid. J Adv Chem 5:830–838
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negm, N.A., Ahmed, S.A., Badr, E.A. et al. Synthesis and Evaluation of Nonionic Surfactants Derived from Tannic Acid as Corrosion Inhibitors for Carbon Steel in Acidic Medium. J Surfact Deterg 18, 989–1001 (2015). https://doi.org/10.1007/s11743-015-1742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1742-2