Abstract
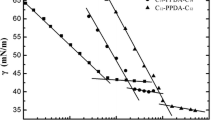
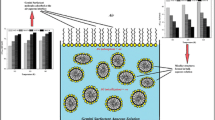
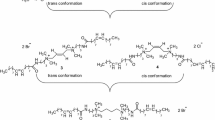
A series of cationic gemini surfactants containing different spacer length were synthesized and analyzed structurally. It was shown that the surface tension (σ) and critical micelle concentration (CMC), which had a maximum for the n-C4H8 spacer depended on the spacer length. The foaming ability and foam stability are high for the gemini surfactants with short spacers (C2H4 to n-C4H8), while longer spacers lead to a distinct decrease of these foam parameters. Foaming properties are discussed in terms of configuration and conformation of a surfactant molecule and in relation to micellization state kinetic.



Similar content being viewed by others
References
Oh SG, Shah DO (1993) The effect of micellar lifetime on the rate of solubilization and detergency in sodium dodecyl sulfate solutions. J Am Oil Chem Soc 70:673–678
Patist A, Jha BK, Oh SG, Shah DO (1999) Importance of micellar relaxation time on detergent properties. J Surf Deterg 2:317–324
Oh SG, Shah DO (1991) Relationship between micellar lifetime and foamability of sodium dodecyl sulfate and sodium dodecyl sulfate/1-hexanol mixtures. Langmuir 7:1316–1318
Trzebicka B, Dworak A, Hawranke J, Kuliszewska E, Hordyjewicz-Baran Z (2013) Chapter 4: Micellization of Gemini Surfactants in Aqueous Solutions. In: Bradburn D, Bittinger T (eds) Micelles: Structural Biochemistry, Formation and Functions & Usage. Nova Science Publishers, Inc., New York, pp 1–48. ISBN 978-1-62948-445-7
Oelschlaeger C, Watson G, Candau SJ, Cates ME (2002) Structural, kinetics, and rheological properties of low ionic strength dilute solutions of a dimeric (gemini) surfactant. Langmuir 18:7265–7272
Ulbricht W, Zana R (2001) Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants: part 8. Pressure-jump study of the kinetics of micellar equilibria in aqueous solutions of alkanediyl-α,ω-bis(dimethyldodecylammonium bromide) surfactants. Coll Surf A 183–185:487–494
Kern F, Lequeux F, Zana R, Candau SJ (1994) Dynamic properties of salt-free viscoelastic micellar solutions. Langmuir 10:1714–1723
Frindi M, Michels B, Levy H, Zana R (1994) Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. Part 4. Ultrasonic absorption studies of amphiphile exchange between micelles and bulk phase in aqueous micellar solution. Langmuir 10:1140–1145
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Coll Interface Sci 248:203–220
Groth C, Nyden M, Holmber K, Kanicky JR, Shah DO (2004) Kinetics of the self-assembly of gemini surfactants. J Surf Deterg 7:247–255
Yang QQ, Hong Q, Somasundaran P (2009) 1H NMR study of micelles formed by mixture of nonionic n-dodecyl-β-d-maltoside and cationic gemini surfactants. J Mol Liq 146:105–111
Manger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Pozniak BP, Kuliszewska E (2013) Competition between substitution and elimination reaction channels from fragmentation ratios of diquaternary ammonium bromide and formate ion pairs. Int J Mass Spectrom 348:29–38
Zana R, Benrraou M, Rueff R (1991) Alkenediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. Part 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 7:1072–1075
Alami E, Beinert G, Marie P, Zana R (1993) Alkenediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. Part 3. Behavior at the air-water interface. Langmuir 9:1465–1467
Tehrani-Bagha AR, Holmberg K (2010) Cationic ester-containing gemini surfactants: physical–chemical properties. Langmuir 26:9276–9282
Laschewsky A, Lunkenheimer K, Rakotoaly RH, Watterbled L (2005) Spacer effects in dimeric cationic surfactants. Coll Polym Sci 283:469–479
Pinoza A, Perez L, Infante MR, Franses EI (2001) Relation of foam stability to solution and surface properties of gemini cationic surfactants derived from arginine. Coll Surf A 189:225–235
Acknowledgments
We kindly acknowledge S. Felsinger for recording NMR measurements and A. Charciarek for performing the foamability tests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kuliszewska, E., Brecker, L. Gemini Surfactants Foam Formation Ability and Foam Stability Depends on Spacer Length. J Surfact Deterg 17, 951–957 (2014). https://doi.org/10.1007/s11743-014-1582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1582-5