Abstract
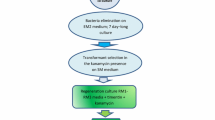
We have optimized a procedure for genetic transformation of a major leafy vegetable crop, Amaranthus tricolor L., using epicotyl explant co-cultivation with Agrobacterium tumefaciens. Two disarmed A. tumefaciens strains EHA 105 and LBA 4404, both carrying the binary plasmid p35SGUSINT harboring the neomycin phosphotransferase II gene (nptII) and the β-glucuronidase gene (gus), were evaluated as vector systems. The former displayed a higher transforming efficiency. Several key factors influencing the transformation events were optimized. The highest percentage of transformed shoots (24.24%) was achieved using hand-pricked epicotyl explants, a 10-min infection period, with 100 μM acetosyringone-pretreated Agrobacterium culture corresponding to OD600 ≅ 0.6 and diluted to 109 cells ml−1, followed by 4 d co-cultivation in the regeneration medium. Putative transformed explants capable of forming shoots were selected on medium supplemented with 75 μg ml−1 kanamycin, and transient as well as stable glucuronidase expression was determined by histochemical analysis. From a total of 48 selected shoot lines derived from independent transformation events with epicotyl explants co-cultivated with EHA 105, 32 showed positive PCR amplification for both the nptII and gus genes. Germ line transformation and transgene stability were evident in progeny of primary transformed plants (T0). Among T1 seedlings of 12 selected transgenic plant lines, kanamycin-resistant and kanamycin-sensitive seedlings segregated in a ratio typical of the Mendelian monohybrid pattern (3:1) as verified by the chi-square (χ 2) test. Southern hybridization of genomic DNA from kanamycin-resistant T1 transgenic segregants to an nptII probe substantiated stable integration of the transgene. Neomycin phosphotransferase (NPTII) activity was detected in leaf protein extracts of selected T1 transgenic plants, thereby confirming stable expression of the nptII gene.







Similar content being viewed by others
References
Ahmed MB, Akhter MS, Hossain M, Islam R, Choudhury TA, Hannan MM, Razvy MA, Ahmad I (2007) An efficient Agrobacterium-mediated genetic transformation method of lettuce (Lactuca sativa L.) with an aphidicidal gene, Pta (Pinellia ternata agglutinin). Middle-East J Sci Res 2:155–160
Allen GC, Hall GEJ, Michalowski S, Newman W, Spiker S, Weissinger AK, Thompson WF (1996) High-level transgene expression in plant cells: effects of a strong scaffold attachment region from tobacco. Plant Cell 8:899–913
Beck E, Ludwig G, Auerswald EA, Reiss B, Schaller H (1982) Nucleotide sequence and exact localization of the neomycin phosphortransferase gene from transposon Tn 5. Gene 19:327–336
Becker D, Kemper E, Schell J, Masterson R (1992) New plant binary vectors with selected markers located proximal to the left T-DNA border. Plant Mol Biol 20:1195–1197
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
Birch RC (1997) Plant transformation: problems and strategies for practical application. Plant Mol Biol 48:297–326
Bresler G, Vaamonde G, Brizzio S (1991) Natural occurrence of zearalenone and toxicogenic fungi in amaranth grain. Intl J Food Microbiol 13:75–80
Butaye KJM, Goderis IJWM, Wouters PFJ, Pues JM-TG, Delauré SL, Broekaert WF, Depicker A, Cammune BPA, De Bolle MFC (2004) Stable high-level transgene expression in Arabidopsis thaliana using gene silencing mutants and matrix attachment regions. Plant J 39:440–449
Chakrabarty R, Viswakarma N, Bhat SR, Kirti PB, Singh BD, Chopra VL (2002) Agrobacterium-mediated transformation of cauliflower: optimization of protocol and development of Bt-transgenic cauliflower. J Biosci 27:495–502
Chugh A, Vikrant MA, Khurana P (2012) A novel approach for Agrobacterium-mediated germ line transformation of Indian bread wheat (Triticum aestivum) and pasta wheat (Triticum durum). J Phytol 4:22–29
De Buck S, De Wilde C, Van Montagu M, Depicker A (2000) T-DNA vector backbone sequences are frequently integrated into the genome of transgenic plants obtained by Agrobacterium-mediated transformation. Mol Breed 6:459–468
Donnarumma F, Paffetti D, Fladung M, Biricolti S, Dieter E, Altosaar I, Vettori C (2011) Transgene copy number estimation and analysis of gene expression levels in Populus spp. transgenic lines. BMC Proc 5(Suppl 7):152
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37
Gendloff EH, Bowen B, Buchholz WG (1990) Quantitation of chloramphenicol acetyl transferase in transgenic tobacco plants by ELISA and correlation with gene copy number. Plant Mol Biol 14:575–583
Gomez KA, Gomez AA (1984) Statistical procedure for agricultural research, 2nd edn. Wiley, New York
Grevelding C, Fantes V, Kemper E, Schell J, Masterson R (1993) Single copy T-DNA insertions in Arabidopsis are the predominant form of integration in root derived transgenics whereas multiple insertions are found in leaf discs. Plant Mol Biol 23:847–860
Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M (1996) Retrotranposons of rice in mutations induced by tissue culture. Proc Natl Acad Sci USA 93:7783–7788
Hobbs SLA, Kpodar P, Delong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformation. Plant Mol Biol 15:851–864
Hobbs SLA, Warketin TD, Delong CMO (1993) Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol Biol 21:17–26
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plant. Transgen Res 2:208–218
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Ji-Hong Z, Hong-Yan Z, Neng-Guo T, Yu-Fang T, Xiao-Yun Z, You-Xiu L (2009) Several methods to detect the inheritance and resistance to the Diamondback Moth in transgenic Chinese cabbage. Afr J Biotechnol 8:2887–2892
Jin RG, Liu YB, Tabashnik BE, Borthakur D (2000) Development of transgenic cabbage (Brassica oleracea var. capitata) for insect resistance by Agrobacterium tumefaciens-mediated transformation. In Vitro Cell Dev Biol Plant 36:231–237
Jofre-Garfias AE, Villegas-Sepúlveda N, Cabrera-Ponce JL, Adame-Alvarez RM, Herrera-Estrella L, Simpson J (1997) Agrobacterium-mediated transformation of Amaranthus hypochondriacus: light- and tissue-specific expression of a pea chlorophyll a/b-binding protein promoter. Plant Cell Rep 16:847–852
Knoll KA, Short KC, Curtis IS, Power JB, Davey MR (1997) Shoot regeneration from cultured root explants of spinach (Spinacia oleracea L.): a system for Agrobacterium transformation. Plant Cell Rep 17:96–101
Kohli A, Twyman RM, Abranches R, Wegel E, Stoger E, Christou P (2003) Transgene integration, organization and interaction in plants. Plant Mol Biol 52:247–258
Kononov ME, Bassuner B, Gelvin SB (1997) Integration of T-DNA binary vector ‘backbone’ sequences into the tobacco genome: evidence for multiple complex patterns of integration. Plant J 11:945–957
Koprek T, Rangel S, McElroy D, Louwerse JD, Williams-Carrier RE, Lemaux PG (2001) Transposon-mediated single-copy gene delivery leads to increased transgene expression in barley. Plant Physiol 125:135–1362
Kumari N, Prakash D (2005) Carotenoids: do they defend against cancer and heart diseases? Inov Intel 40:24–30, gaind@nrdcindia.com
Li X, Volrath SL, Nicholl DBG, Chilcott CE, Johnson MA, Ward ER, Law MD (2003) Development of protoporphyrinogen oxidase as an efficient selection marker for Agrobacterium tumefaciens-mediated transformation of maize. Plant Physiol 133:736–747
Matzke MA, Matzke AJM, Eggleston WB (1996) Paramutation and transgene silencing: a common response to invasive DNA? Trends Plant Sci 1:382–388
Metcalf RL, Metcalf RA (1993) Insects injurious to vegetable gardens and truck crops. In: Destructive and useful insects: their habits and control, 5th edn. McGraw-Hill, New York
Metz TD, Dixit R, Earle ED (1995) Agrobacterium tumefaciens-mediated transformation of broccoli (Brassica oleracea var. italica) and cabbage (B. oleracea var. capitata). Plant Cell Rep 15:287–292
Meyer P, Lohuis MT, van Blockland R, Heidmann I, Niedenhof I (1996) The role of DNA methylation in transgene silencing in plants. In: Grierson D, Lyett GW, Tucker GA (eds) Mechanisms and applications of gene silencing. Nottingham University Press, Sheffield, pp 43–48
Mohapatra U, McCabe MS, Power JB, Schepers F, Vander Arend A, Davey MR (1999) Expression of the bar gene confers herbicide resistance in transgenic lettuce. Transgen Res 8:33–44
Murashige T, Skoog F (1962) A revised medium for rapid bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Muskens MW, Vissers AP, Mol JN, Kooter JM (2000) Role of inverted DNA repeats in transcriptional and post-transcriptional gene silencing. Plant Mol Biol 43:243–260
Nehra NS, Stushnoff C, Kartha KK (1990) Regeneration of plants from immature leaf-derived callus of strawberry (Fragaria × Ananassa). Plant Sci 66:119–126
Pal A (2008) Genetic transformation of Amaranthus species using Ri and Ti plasmid vectors. Ph.D. Thesis. Utkal University, Bhubaneswar India, 183 pp
Pal A, Swain SS, Mukherjee AK, Chand PK (2013) Agrobacterium pRi TL-DNA rolB and TR-DNA opine genes transferred to the spiny amaranth (Amaranthus spinosus L.)—a nutraceutical crop. Food Technol Biotechnol 51(1) (in press)
Reddy MS, Dinkins RD, Collins GB (2003) Gene silencing in transgenic soybean plants transformed via particle bombardment. Plant Cell Rep 21:676–683
Reiss B, Sprengel R, Schaller H (1984) Protein fusions with the kanamycin resistance gene from transposon Tn5. EMBO J 3:3317–3322
Shyr YYJ, Hepburn AG, Widholm JM (1992) Glyphosate selected amplification of the 5-enolpyruvylshikimate-3-phosphate synthase gene in cultured carrot cells. Mol Gen Genet 232:377–382
Sparrow PAC, Dale PJ, Irwin JA (2004) The use of phenotypic markers to identify Brassica oleracea genotypes for routine high through-put Agrobacterium-mediated transformation. Plant Cell Rep 23:64–70
Stoger E, Williams S, Keen D, Christou P (1998) Molecular characteristics of transgenic wheat and the effect on transgene expression. Transgenic Res 7:463–471
Subramanyam K, Subramanyam K, Sailaja KV, Srinivasulu M, Lakshmidevi K (2011) Highly efficient Agrobacterium-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. Plant Cell Rep. doi:10.1007/s00299-010-0996-4
Swain SS, Sahu L, Barik DP, Chand PK (2010) Agrobacterium × plant factors influencing transformation of ‘Joseph's coat’ (Amaranthus tricolor L.). Sci Hortic 125:461–468
Taiwo MA, Owolabi AT (2004) Comparative study of Amaranthus leaf mottle virus genus Potyvims and Amaranthus mosaic virus. J Sci Res Dev 9:63–74
Tenea G, Cucu N (2006) The influence of T-DNA copy numbers on gene expression in primary tranformants Atropa belladonna plants. Roum Biotechnol Lett 11:2661–2667
The Wealth of India (1948) Raw materials, vol I. C.S.I.R, New Delhi, pp 66–67
Thiruvengadam M, Chung IM (2011) Establishment of an efficient Agrobacterium tumefaciens-mediated leaf disc transformation of spine gourd (Momordica dioica Roxb. ex Willd). Afr J Biotechnol 10:19337–19345
Tsukazaki H, Kuginuki Y, Aida R, Suzuki T (2002) Agrobacterium- mediated transformation of a doubled haploid line of cabbage. Plant Cell Rep 21:257–262
Tzfira T, Lacroix B, Citovsky V (2004) Agrobacterium T-DNA integration: molecules and models. Trends Genet 20:375–383
Valimareanu S (2010) Leaf disk transformation of Lactuca sativa using Agrobacterium tumefaciens. Not Bot Hort Agrobot Cluj 38:181–186
van der Hoeven C, Dietz A, Landsmann J (1994) Variability in organ-specific gene expression in transgenic tobacco plants. Transgenic Res 3:159–165
Vancanneyt G, Schmidt R, O’Connor-Shanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220:245–250
Wang GL, Fang HJ (1998) Mechanism and technology of plant genetic engineering. Science, Beijing
Wang MB, Waterhouse PM (2000) High-efficiency silencing of a beta-glucuronidase gene in rice is correlated with repetitive transgene structure but is independent of DNA methylation. Plant Mol Biol 43:67–82
Zhang HX, Zeevart JAD (1999) An efficient Agrobacterium tumefaciens-mediated transformation and regeneration system for cotyledons of spinach (Spinacia oleracea L.). Plant Cell Rep 18:640–645
Acknowledgments
We wish to thank Dr. Jatindra K. Nayak, Professor of English, Utkal University, Bhubaneswar, India for critically reading the manuscript and making useful changes in the text language.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Pal, A., Swain, S.S., Das, A.B. et al. Stable germ line transformation of a leafy vegetable crop amaranth (Amaranthus tricolor L.) mediated by Agrobacterium tumefaciens . In Vitro Cell.Dev.Biol.-Plant 49, 114–128 (2013). https://doi.org/10.1007/s11627-013-9489-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9489-9