Abstract
Purpose
To determine the maximum tolerated dose (MTD) of [131I]tositumomab in patients with refractory/recurrent Hodgkin lymphoma (HL) and to preliminarily determine if [131I]tositumomab has activity against HL and if positron emission tomography (PET) with 2-deoxy-2-[18F]fluoro-D-glucose ([18F]DG) performed 6 weeks post-therapy predicted 12-week response.
Procedures
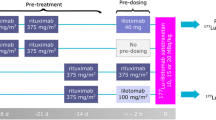
Separate dose-finding studies were performed for patients with and without prior transplant. A single therapeutic total body radiation dose (TBD) of [131I]tositumomab was administered. TBD was escalated/de-escalated based on dose-limiting hematologic toxicity (DLT) using a modified continual reassessment method. [18F]DG-PET/CT scans were performed at baseline and 6 and 12 weeks post therapy.
Results
Twelve patients (nine classical HL, three lymphocyte-predominant [LP] HL) completed two dosing levels (n = 3 each) in the post-transplant (55 cGy, 79 cGy) and no transplant (75 cGy, 87 cGy) groups. Hematologic toxicities were common and transient. Twelve weeks after [131I]tositumomab, 10 patients progressed and two with LPHL achieved complete response. [18F]DG-PET/CT at 6 weeks post therapy appeared more predictive than CT at 6 weeks of a response at 12 weeks.
Conclusions
Tositumomab and [131I]tositumomab was well-tolerated in patients with relapsed/refractory HL. Complete responses in LPHL support a therapeutic effect in this subtype. Early metabolic response assessments by [18F]DG-PET in HL after radioimmunotherapy appear to be more predictive than purely anatomic assessments.


Similar content being viewed by others
References
Marafioti T, Hummel M, Anagnostopoulos I et al (1997) Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med 337:453–458
Foss HD, Reusch R, Demel G et al (1999) Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin's disease provides further evidence for its B-cell origin. Blood 94:3108–3113
Watanabe K, Yamashita Y, Nakayama A et al (2000) Varied B-cell immunophenotypes of Hodgkin/Reed-Sternberg cells in classic Hodgkin's disease. Histopathology 36:353–361
Advani RH, Horning SJ, Hoppe RT et al (2014) Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 32:912–918
Eichenauer DA, Fuchs M, Pluetschow A et al (2011) Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 118:4363–4365
Karamouzis MV, Apostolikas N, Georganta C et al (2004) Case of relapsed CD20(+) mixed-cellularity Hodgkin disease treated with sequential rituximab and radiotherapy. Am J Hematol 77:418–419
Younes A, Romaguera J, Hagemeister F et al (2003) A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer 98:310–314
Jones RJ, Gocke CD, Kasamon YL et al (2009) Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood 113:5920–5926
Davis TA, Kaminski MS, Leonard JP et al (2004) The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res 10:7792–7798
Witzig TE, Gordon LI, Cabanillas F et al (2002) Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 20:2453–2463
Brown RS, Kaminski MS, Fisher SJ et al (1997) Intratumoral microdistribution of [131I]MB-1 in patients with B-cell lymphoma following radioimmunotherapy. Nucl Med Biol 24:657–663
Ekstrand BC, Lucas JB, Horwitz SM et al (2003) Rituximab in lymphocyte-predominant Hodgkin disease: results of a phase 2 trial. Blood 101:4285–4289
Younes A, Wong F (2009) Experience with 90Y-ibritumomab tiuxetan for relapsed classical Hodgkin lymphoma. Ann Oncol 20:1147–1148
Jacene HA, Filice R, Kasecamp W, Wahl RL (2007) Comparison of 90Y-ibritumomab tiuxetan and 131I-tositumomab in clinical practice. J Nucl Med 48:1767–1776
Kaminski MS, Estes J, Zasadny KR et al (2000) Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood 96:1259–1266
Wiseman GA, White CA, Stabin M et al (2000) Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin's lymphoma. E J Nucl Med Mol Imaging 27:766–777
Piantadosi S, Fisher JD, Grossman S (1998) Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol 41:429–436
Lister TA, Crowther D, Sutcliffe SB et al (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 7:1630–1636
Jacene HA, Filice R, Kasecamp W, Wahl RL (2009) 18F-FDG PET/CT for monitoring the response of lymphoma to radioimmunotherapy. J Nucl Med 50:8–17
Juweid ME, Wiseman GA, Vose JM et al (2005) Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol 23:4652–4661
Iasonos A, Wilton AS, Riedel ER, Seshan VE, Spriggs DR (2008) A comprehensive comparison of the continual reassessment method to the standard 3 + 3 dose escalation scheme in phase I dose-finding studies. ClinTrials 5:465–477
Fisher RI, Kaminski MS, Wahl RL et al (2005) Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin’s lymphomas. J Clin Oncol 23:7565–7573
Kaminski MS, Tuck M, Estes J et al (2005) 131 I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med 352:441–449
Kaminski MS, Zasadny KR, Francis IR et al (1996) Iodine-131-anti-B1 radioimmunotherapy for B-cell lymphoma. J Clin Oncol 14:1974–1981
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Kasamon YL, Jacene HA, Gocke CD et al (2012) Phase 2 study of rituximab-ABVD in classical Hodgkin lymphoma. Blood 119:4129–4132
Younes A, Oki Y, McLaughlin P et al (2012) Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood 119:4123–4128
Torizuka T, Zasadny KR, Kison PV et al (2000) Metabolic response of non-Hodgkin's lymphoma to 131I-anti-B1 radioimmunotherapy: evaluation with FDG PET. J Nucl Med 41:999–1005
Gopal AK, Press OW, Wilbur SM et al (2008) Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood 112:830–835
Buchsbaum DJ, Wahl RL, Glenn SD et al (1992) Improved delivery of radiolabeled anti-B1 monoclonal antibody to Raji lymphoma xenografts by predosing with unlabeled anti-B1 monoclonal antibody. Cancer Res 52:637–642
Horning SJ, Younes A, Jain V et al (2005) Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J Clin Oncol 23:712–719
Witzig TE, Flinn IW, Gordon LI et al (2002) Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol 20:3262–3269
Stern M, Herrmann R, Rochlitz C et al (2005) A case of post-transplant lymphoproliferative disease presenting as CD20-expressing, Epstein-Barr-virus positive Hodgkin lymphoma. Eur J Haematol 74:267–270
Leahy MF, Seymour JF, Hicks RJ, Turner JH (2006) Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin's lymphoma. J Clin Oncol 24:4418–4425
Schaefer NG, Huang P, Buchanan JW, Wahl RL (2011) Radioimmunotherapy in non-Hodgkin lymphoma: opinions of nuclear medicine physicians and radiation oncologists. J Nucl Med 52:830–838
Schaefer NG, Ma J, Huang P et al (2010) Radioimmunotherapy in non-Hodgkin lymphoma: opinions of U.S. medical oncologists and hematologists. J Nucl Med 51:987–994
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Acknowledgments
This study was supported by GlaxoSmithKline and Johns Hopkins SPORE in Lymphoma grant #1 P50 CA96888-04 (PI Ambinder). The role of GlaxoSmithKline was financial support of the study. Study design, collection, analysis and interpretation of data, preparation of the manuscript and decision for publication were the roles of the investigators. The authors thank Mrs. Judy Buchanan for her assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Funding
This study was supported by GlaxoSmithKline and Johns Hopkins SPORE in Lymphoma grant #1 P50 CA96888-04 (PI Ambinder).
Rights and permissions
About this article
Cite this article
Jacene, H., Crandall, J., Kasamon, Y.L. et al. Initial Experience with Tositumomab and I-131-Labeled Tositumomab for Treatment of Relapsed/Refractory Hodgkin Lymphoma. Mol Imaging Biol 19, 429–436 (2017). https://doi.org/10.1007/s11307-016-1019-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-1019-9