Abstract
Aims
Stressful conditions, such as heat and hypoxia stresses, could enhance glucosinolate metabolism, but impacted plant growth and productivity. It is very important to identify the differences in physiological and proteomic changes in broccoli sprouts under the stresses of heat, hypoxia and heat plus hypoxia.
Methods
Physiological properties, antioxidant enzyme activity and glucosinolates and sulforaphane were studied. The iTRAQ technique was applied to identify the differentially abundant proteins.
Results
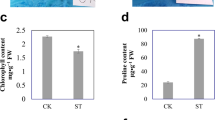
A total of 145, 92 and 105 proteins in broccoli sprouts showed differential relative abundance under the stresses of heat, hypoxia and heat plus hypoxia, respectively. These proteins were mainly involved in energy, defense/stress, carbohydrate catabolism and protein biosynthesis. Compared with heat- and hypoxia-stress, heat plus hypoxia stress resulted in 102 and 17 differentially abundant proteins, respectively.
Conclusions
Heat and heat plus hypoxia stresses might modulate stress-triggered ROS homeostasis and MDA by activating heat shock proteins and antioxidant enzymes as well as by inducing of glucosinolates and sulforaphane. Hypoxia stress induced antioxidant enzymes and glucosinolates metabolism to strengthen the defense system in broccoli sprouts. Heat plus hypoxia stress did not have the synergistic effect of heat and hypoxia both in physiological changes and in proteomic analysis; and it was similar to hypoxia stress.








Similar content being viewed by others
References
Bartwal A, Mall R, Lohani P, Guru SK, Arora S (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Reg 32:216–232
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Chaitanya K, Sundar D, Masilamani S, Reddy AR (2002) Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Reg 36:175–180
Chang IF (2005) Proteomic characterization of evolutionarily conserved and variable proteins of Arabidopsis cytosolic ribosomes. Plant Physiol 137:848–862
Chang WW, Huang L, Shen M, Webster C, Burlingame AL, Roberts JK (2000) Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol 122:295–318
Chen Y, Pang Q, Dai S, Wang Y, Chen S, Yan X (2011) Proteomic identification of differentially expressed proteins in Arabidopsis in response to methyl jasmonate. J Plant Physiol 168:995–1008
Christou A, Manganaris GA, Fotopoulos V (2014) Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ Exp Bot 107:46–54
de Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Duncan RF, Hershey J (1989) Protein synthesis and protein phosphorylation during heat stress, recovery, and adaptation. J Cell Biol 109:1467–1481
Fahey JW, Zhang Y, Talalay P (1997) Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. P Natil Acad Sci 94:10367–10372
Galvis MLE, Marttila S, Håkansson G, Forsberg J, Knorpp C (2001) Heat stress response in pea involves interaction of mitochondrial nucleoside diphosphate kinase with a novel 86-kilodalton protein. Plant Physiol 126:69–77
Giavalisco P, Wilson D, Kreitler T, Lehrach H, Klose J, Gobom J, Fucini P (2005) High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome. Plant Mol Biol 57:577–591
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gu Z, Guo Q, Gu Y (2012) Factors influencing glucoraphanin and sulforaphane formation in Brassica plants: a review. J Integr Agri 11:1804–1816
Guo L, Yang R, Wang Z, Guo Q, Gu Z (2014a) Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int J Food Sci Nutr 65:476–481
Guo L, Yang R, Wang Z, Guo Q, Gu Z (2014b) Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J Funct Foods 9:70–77
Guo L, Yang R, Zhou Y, Gu Z (2016a) Heat and hypoxia stresses enhance the accumulation of aliphatic glucosinolates and sulforaphane in broccoli sprouts. Eur Food Res Technol 242:107–116
Guo L, Yang R, Gu Z (2016b) Cloning of genes related to aliphatic glucosinolate metabolism and the mechanism of sulforaphane accumulation in broccoli sprouts under jasmonic acid treatment. J Sci Food Agri 96:4329–4336
Jagadish S, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ (2010) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J Exp Bot 61:143–156
Jahangir M, Abdel-Farid IB, Kim HK, Choi YH, Verpoorte R (2009) Healthy and unhealthy plants: the effect of stress on the metabolism of Brassicaceae. Environ Exp Bot 67:23–33
Juge N, Mithen R, Traka M (2007) Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 64:1105–1127
Kissen R, Bones AM (2009) Nitrile-specifier proteins involved in glucosinolate hydrolysis in Arabidopsis thaliana. J Biol Chem 284:12057–12070
Konishi H, Yamane H, Maeshima M, Komatsu S (2004) Characterization of fructose-bisphosphate aldolase regulated by gibberellin in roots of rice seedling. Plant Mol Biol 56:839–848
Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J Proteome 74:1301–1322
Lee DG, Ahsan N, Lee SH, Kang KY, Bahk JD, Lee IJ, Lee BH (2007) A proteomic approach in analyzing heat-responsive proteins in rice leaves. Proteomics 7:3369–3383
Lepistö A, Kangasjärvi S, Luomala EM, Brader G, Sipari N, Keränen M, Keinänen M, Rintamäki E (2009) Chloroplast NADPH-thioredoxin reductase interacts with photoperiodic development in Arabidopsis. Plant Physiol 149:1261–1276
Liu GT, Ma L, Duan W, Wang BC, Li JH, Xu HG, Yan XQ, Yan BF, Li SH, Wang LJ (2014) Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC Plant Biol 14:110
Madhava Rao K, Sresty T (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Matusheski NV, Juvik JA, Jeffery EH (2004) Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochem 65:1273–1281
Matusheski NV, Swarup R, Juvik JA, Mithen R, Bennett M, Jeffery EH (2006) Epithiospecifier protein from broccoli (Brassica oleracea L. Ssp. italica) inhibits formation of the anticancer agent sulforaphane. J Agri Food Chem 54:2069–2076
Mayfield SP, Bennoun P, Rochaix J (1987) Expression of the nuclear encoded OEE1 protein is required for oxygen evolution and stability of photosystem II particles in Chlamydomonas reinhardtii. EMBO J 6:313
McKeon TA, Fernández-Maculet JC, Yang SF (1995) Biosynthesis and metabolism of ethylene. In: Davies PJ (ed) Plant hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 118–139
Mewis I, Schreiner M, Nguyen CN, Krumbein A, Ulrichs C, Lohse M, Zrenner R (2012) UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol 53:1546–1560
Pereira FMV, Rosa E, Fahey JW, Stephenson KK, Carvalho R, Aires A (2002) Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J Agri Food Chem 50:6239–6244
Pérez-Balibrea S, Moreno DA, García-Viguera C (2008) Influence of light on health-promoting phytochemicals of broccoli sprouts. J Sci Food Agri 88:904–910
Pérez-Balibrea S, Moreno DA, García-Viguera C (2011) Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem 129:35–44
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Schonhof I, Kläring HP, Krumbein A, Schreiner M (2007) Interaction between atmospheric CO2 and glucosinolates in broccoli. J Chem Ecol 33:105–114
Shahak Y, Crowther D, Hind G (1981) The involvement of ferredoxin-NADP+ reductase in cyclic electron transport in chloroplasts. BBA-Bioenergetics 636:234–243
Shakeel SN, Aman S, Haq NU, Heckathorn SA, Luthe D (2013) Proteomic and transcriptomic analyses of Agave americana in response to heat stress. Plant Mol Biol Rep 31:840–851
Sønderby IE, Geu-Flores F, Halkier BA (2010) Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci 15:283–290
Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. BBA-Gene Struct Expr 1577:1–9
Tiwari A, Kumar P, Singh S, Ansari S (2005) Carbonic anhydrase in relation to higher plants. Photosynthetica 43:1–11
Traka M, Mithen R (2009) Glucosinolates, isothiocyanates and human health. Phytochem Rev 8:269–282
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang X, Dinler BS, Vignjevic M, Jacobsen S, Wollenweber B (2015) Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci 230:33–50
Zhang Y, Xu L, Zhu X, Gong Y, Xiang F, Sun X, Liu L (2013) Proteomic analysis of heat stress response in leaves of radish (Raphanus sativus L.). Plant Mol Biol Rep 31:195–203
Acknowledgements
This work was supported by China Postdoctoral Science Foundation (2015 M570455). and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric J.W. Visser.
Electronic supplementary material
Supplementary Table S1
(DOCX 58 kb)
Supplementary Table S2
(DOCX 43 kb)
Supplementary Table S3
(DOCX 30 kb)
Supplementary Table S4
(DOCX 32 kb)
Supplementary Table S5
(DOCX 40 kb)
Supplementary Table S6
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Guo, L., Gu, Z., Jin, X. et al. iTRAQ - based proteomic and physiological analyses of broccoli sprouts in response to the stresses of heat, hypoxia and heat plus hypoxia. Plant Soil 414, 355–377 (2017). https://doi.org/10.1007/s11104-016-3132-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3132-6