Abstract
Navel orange (Citrus sinensis [L.] Osbeck) fruit surfaces contain substantial quantities of cuticular waxes, which have important eco-physiological roles, such as water retention and pathogen defense. The wax constituents of ripe navel orange have been studied in various reports, while the wax changes occurring during fruit development and the molecular mechanism underlying their biosynthesis/export have not been investigated. Recently, we reported a spontaneous bud mutant from the wild-type (WT) ‘Newhall’ Navel orange. This mutant displayed unusual glossy fruit peels and was named ‘glossy Newhall’ (MT). In this study, we compared the developmental profiles of the epicuticular and intracuticular waxes on the WT and MT fruit surfaces. The formation of epicuticular wax crystals on the navel orange surface was shown to be dependent on the accumulation of high amounts of aliphatic wax components with trace amounts of terpenoids. In sharp contrast, the underlying intracuticular wax layers have relatively low concentrations of aliphatic wax components but high concentrations of cyclic wax compounds, especially terpenoids at the late fruit developmental stages. Our work also showed that many genes that are involved in wax biosynthesis and export pathways were down-regulated in MT fruit peels, leading to a decrease in aliphatic wax component amounts and the loss of epicuticular wax crystals, ultimately causing the glossy phenotype of MT fruits.





Similar content being viewed by others
References
Aarts MG, Keijzer CJ, Stiekema WJ, Pereira A (1995) Molecular characterization of the CER1 gene of arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 7:2115–2127
Albrigo LG (1972) Ultrastructure of cuticular surfaces and stomata of developing leaves and fruit of the Valencia oranges. J Am Chem Soc 97:761–765
Baker EA, Procopiou J, Hunt GM (1975) The cuticles of citrus species. Composition of leaf and fruit waxes. J Sci Food Agric 26:1093–1101
Baker EA, Bukovac J, Hunt GM (1982) Composition of tomato fruit cuticle as related to fruit growth and development. In: Cutler DF, Alvin KL, Price CE (eds) The plant cuticle. Academic Press, London, pp 33–44
Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126:237–260
Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33:75–86
Ben-Yehoshua S, Burg SP, Young R (1985) Resistance of citrus fruit to mass transport of water vapor and other gases. Plant Physiol 79:1048–1053
Bernard A, Joubès J (2013) Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Prog Lipid Res 52:110–129
Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J (2012) Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very long-chain alkane synthesis complex. Plant Cell 24:3106–3118
Bird DA (2008) The role of ABC transporters in cuticular lipid secretion. Plant Sci 174:563–569
Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L (2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52:485–498
Blacklock BJ, Jaworski JG (2006) Substrate specificity of Arabidopsis 3-ketoacyl-CoA synthases. Biochem Biophys Res Commun 6:583–590
Bourdenx B, Bernard A, Domergue F, Pascal S, Léger A, Roby D, Pervent M, Vile D, Haslam RP, Napier JA, Lessire R, Joubès J (2011) Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol 156:29–45
Buschhaus C, Herz H, Jetter R (2007) Chemical composition of the epicuticular and intracuticular wax layers on the adaxial side of Ligustrum vulgare leaves. New Phytol 176:311–316
Cajuste JF, González-Candelas L, Veyrat A, García-Breijo FJ, Reig-Armiñana J, Lafuente MT (2010) Epicuticular wax content and morphology as related to ethylene and storage performance of ‘Navelate’orange fruit. Postharvest Biol Technol 55:29–35
Chen X, Goodwin SM, Boroff VL, Liu X, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15:1170–1185
Denic V, Weissman JS (2007) A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 130:663–677
Dore A, Molinu MG, Venditti T, D’hallewin G (2009) Immersion of lemons into imazalil mixtures heated at 50 °C alters the cuticle and promotes permeation of imazalil into rind wounds. J Agric Food Chem 57:623–631
El-Otmani M, Coggins CWJ (1985) Fruit age and growth regulator effects on the quantity and structure of the epicuticular wax of Washington navel orange fruit. J Am Soc Hortic Sci 110:371–378
El-Otmani M, Coggins CWJ, Eaks IL (1986) Fruit age and gibberellic acid effect on epicuticular wax accumulation, respiration, and internal atmosphere of navel orange fruit. J Am Soc Hortic Sci 111:228–232
Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12:2001–2008
Freeman E, Albrigo LG, Biggs RH (1979) Ultrastructure and chemistry of cuticular waxes of developing Citrus leaves and fruits. J Am Soc Hortic Sci 104:801–808
Gniwotta F, Vogg G, Gartmann V, Carver TL, Riederer M, Jetter R (2005) What do microbes encounter at the plant surface? Chemical composition of pea leaf cuticular waxes. Plant Physiol 139:519–530
Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM (2000) The HIC signalling pathway links CO2 perception to stomatal development. Nature 408:713–716
Hooker TS, Millar AA, Kunst L (2002) Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol 129:1568–1580
Jenks MA, Tuttle HA, Eigenbrode SD, Feldmann KA (1995) Leaf epicuticular waxes of the eceriferum mutants in Arabidopsis. Plant Physiol 108:369–377
Jetter R, Schaffer S (2001) Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol 126:1725–1737
Jetter R, Schäffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23:619–628
Jetter R, Kunst L, Samuels AL (2006) Composition of plant cuticular waxes. In: Riederer M, Müller C (eds) Biology of the plant Cuticle. Blackwell, Oxford, pp 145–181
Joubès J, Raffaele S, Bourdenx B, Garcia C, Laroche-Traineau J, Moreau P, Domergue F, Lessire R (2008) The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression profiling. Plant Mol Biol 67:547–566
Koch K, Ensikat HJ (2008) The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39:759–772
Koornneef M, Hanhart CJ, Thiel F (1989) A genetic and phenotypic description of eceriferum (cer) mutants in Arabidopsis thaliana. J Hered 80:118–122
Kunst L, Samuels L (2009) Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol 12:721–727
Kunst L, Clemens S, Hooker T (2000) Expression of the wax-specific condensing enzyme CUT1 in Arabidopsis. Biochem Soc T 28:651–654
Lee SB, Jung SJ, Go YS, Kim HU, Kim JK, Cho HJ, Park OK, Suh MC (2009) Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J 60:462–475
Liu DC, Zeng Q, Ji QX, Liu CF, Liu SB, Liu Y (2012) A comparison of the ultrastructure and composition of fruits’ cuticular wax from the wild-type ‘Newhall’ navel orange (Citrus sinensis [L.] Osbeck cv. Newhall) and its glossy mutant. Plant Cell Rep 31:2239–2246
Liu DC, Zeng Q, Liu Y, Wu Q, Wang YC, Liu SB (2013) Study on the chromatism index variation of fruit peel from the ‘Newhall’ navel orange and its glossy mutant during the fruit development. J Fruit Sci 30:914–917
Lü S, Zhao H, Parsons EP, Xu C, Kosma DK, Xu X, Chao D, Lohrey G, Bangarusamy DK, Wang G, Bressan RA, Jenks MA (2011) The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme a carboxylase in the biosynthesis of cuticular waxes by Arabidopsis. Plant Physiol 157:1079–1092
Luo B, Xue X, HuW Wang L, Chen X (2007) An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol 48:1790–1802
McDonald RE, Nordby HE, McCollum TG (1993) Epicuticular wax morphology and composition are related to grapefruit chilling injury. HortScience 28:311–312
Millar AA, Kunst L (1997) Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J 12:121–131
Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L (1999) CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 11:825–838
Nordby HE, McDonald RE (1994) Friedelin, the major component of grapefruit epicuticular wax. J Agric Food Chem 42:708–713
Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, Schreiber L, Chory J, Aharoni A (2007) The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 145:1345–1360
Peschel S, Franke R, Schreiber L, Knoche M (2007) Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 68:1017–1025
Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004) Plant cuticular lipid export requires an ABC transporter. Science 306:702–704
Pollard M, Beisson F, Li Y, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246
Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ (2000) FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. P Natl Acad Sci USA 97:1311–1316
Rashotte A, Jenks M, Ross A, Feldmann K (2004) Novel eceriferum mutants in Arabidopsis thaliana. Planta 219:5–13
Rowland O, Domergue F (2012) Plant fatty acyl reductases: enzymes generating fatty alcohols for protective layers with potential for industrial applications. Plant Sci 193–194:28–38
Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L (2006) CER4 encodes an alcohol-forming fatty acyl-coenzyme a reductase involved in cuticular wax production in Arabidopsis. Plant Physiol 142:866–877
Rowland O, Lee R, Franke R, Schreiber L, Kunst L (2007) The CER3 wax biosynthetic gene from Arabidopsis thaliana is allelic to WAX2/YRE/FLP1. FEBS Lett 581:3538–3544
Sakuradani E, Zhao L, Haslam TM, Kunst L (2013) The CER22 gene required for the synthesis of cuticular wax alkanes in Arabidopsis thaliana is allelic to CER1. Planta 237:731–738
Sala JM (2000) Content, chemical composition and morphology of epicuticular wax of Fortune mandarin fruits in relation to peel pitting. J Sci Food Agr 80:1887–1894
Sala JM, Lafuente T, Cufiat P (1992) Content and chemical composition of epicuticular wax of ‘Navelina’ oranges and ‘Satsuma’ mandarins as related to rindstaining of fruit. J Sci Food Agric 59:489–495
Samuels L, Kunst L, Jetter R (2008) Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol 59:683–707
Sasaki Y, Nagano Y (2004) Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci Biotech Bioch 68:1175–1184
Schirra M, D’hallewin G (1997) Storage performance of fortune mandarins following hot water dips. Postharvest Biol Technol 10:229–237
Schirra M, D’hallewin G, Ben-Yehoshua S, Fallik E (2000) Host–pathogen interactions modulated by heat treatment. Postharvest Biol Technol 21:71–85
Todd J, Post-Beittenmiller D, Jaworski JG (1999) KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17:119–130
Turrell FM (1946) Tables of surfaces and volumes of spheres and of prolate and oblate spheroids, and spheroidal coeffcients. University of California Press, Berkeley
Vogg G, Fischer S, Leide J, Emmanuel E, Jetter R, Levy AA, Riederer M (2004) Tomato fruit cuticular waxes and their effects on transpiration barrier properties: functional characterization of a mutant deficient in a very-long-chain fatty acid beta-ketoacyl-CoA synthase. J Exp Bot 55:1401–1410
Wang J, Hao H, Liu R, Ma Q, Xu J, Chen F, Cheng Y, Deng X (2014) Comparative analysis of surface wax in mature fruits between Satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem 153:177–185
Wen M, Buschhaus C, Jetter R (2006) Nanotubules on plant surfaces: chemical composition of epicuticular wax crystals on needles of Taxus baccata L. Phytochemistry 67:1808–1817
Yanai Y, Kawasaki T, Shimada H, Wurtele ES, Nikolau BJ, Ichikawa N (1995) Genomic organization of 251 kDa acetyl-CoA carboxylase genes in Arabidopsis: tandem gene duplication has made two differentially expressed isozymes. Plant Cell Physiol 36:779–787
Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11:2187–2202
Zhang JZ, Li ZM, Liu L, Mei L, Yao JL, Hu CG (2008) Identification of early-flower-related ESTs in an early-flowering mutant of trifoliate orange (Poncirus trifoliata L. Raf.) by suppression subtractive hybridization and macroarray analysis. Tree Physiol 28:1449–1457
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Numbers 31160384 and 31460511) and the Jiangxi Provincial Natural Science Foundation (Number 20142BAB204008).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
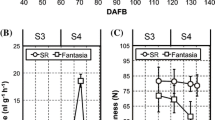
The coverage of various epicuticular wax fractions of wild-type (WT) and glossy (MT) Newhall navel orange fruits at five developmental stages. The genotype of each sample is shown in the upper right of the charts. The values are shown as the mean ± SE with three independent experiments. Single and double asterisks indicate significance at the P < 0.05 and P < 0.01 levels (TIFF 22908 kb)
Fig. 2
Relative epicuticular (a) and intracuticular (b) wax compositions of wild-type (WT) and glossy (MT) Newhall navel orange fruits of five different developmental stages (TIFF 2261 kb)
Fig. 3
The coverage of various intracuticular wax fractions of wild-type (WT) and glossy (MT) Newhall navel orange fruits at five developmental stages. The genotype of each sample is shown in the upper right of the charts. The values are shown as the mean ± SE with three independent experiments. Single and double asterisks indicate significance at the P < 0.05 and P < 0.01 levels (TIFF 23061 kb)
Rights and permissions
About this article
Cite this article
Liu, D., Yang, L., Zheng, Q. et al. Analysis of cuticular wax constituents and genes that contribute to the formation of ‘glossy Newhall’, a spontaneous bud mutant from the wild-type ‘Newhall’ navel orange. Plant Mol Biol 88, 573–590 (2015). https://doi.org/10.1007/s11103-015-0343-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0343-9