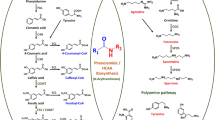
Abstract
Two fructan hydrolases were previously reported to exist in Jerusalem artichoke (Helianthus tuberosus) and one native fructan-β-fructosidase (1-FEH) was purified to homogeneity by SDS-PAGE, but no corresponding cDNA was cloned. Here, we cloned two full-length 1-FEH cDNA sequences from Jerusalem artichoke, named Ht1-FEH I and Ht1-FEH II, which showed high levels of identity with chicory 1-FEH I and 1-FEH II. Functional characterization of the corresponding recombinant proteins in Pichia pastoris X-33 demonstrated that both Ht1-FEHs had high levels of hydrolase activity towards β(2,1)-linked fructans, but low or no activity towards β(2,6)-linked levan and sucrose. Like other plant FEHs, the activities of the recombinant Ht1-FEHs were greatly inhibited by sucrose. Real-time quantitative PCR analysis showed that Ht1-FEH I transcripts accumulated to high levels in the developing leaves and stems of artichoke, whereas the expression levels of Ht1-FEH II increased in tubers during tuber sprouting, which implies that the two Ht1-FEHs play different roles. The levels of both Ht1-FEH I and II transcript were significantly increased in the stems of NaCl-treated plants. NaCl treatment also induced transcription of both Ht1-FEHs in the tubers, while PEG treatments slightly inhibited the expression of Ht1-FEH II in tubers. Analysis of sugar-metabolizing enzyme activities and carbohydrate concentration via HPLC showed that the enzyme activities of 1-FEHs were increased but the fructose content was decreased under NaCl and PEG treatments. Given that FEH hydrolyzes fructan to yield Fru, we discuss possible explanations for the inconsistency between 1-FEH activity and fructan dynamics in artichokes subjected to abiotic stress.








Similar content being viewed by others
Abbreviations
- 1-FFT:
-
Fructan:fructan 1-fructosyltransferase
- 1F-F:
-
1F-Fructofuranosylnystose
- 1-SST:
-
Sucrose:sucrose 1-fructosyltransferase
- 1-K:
-
1-Kestose
- 6G-FFT:
-
Fructan:fructan 6G-fructosyltransferase
- 6-SFT:
-
Sucrose:fructan 6-fructosyltransferase
- CK:
-
Control
- DP:
-
Degree of polymerization
- DW:
-
Dry weight
- FEH:
-
Fructan exohydrolase
- FW:
-
Fresh weight
- Fru:
-
Fructose
- Glc:
-
Glucose
- HPLC:
-
High performance liquid chromatography
- INV:
-
Invertase
- LC–MS:
-
Liquid chromatography–mass spectrometry
- Nys:
-
Nystose
- qPCR:
-
Quantitative PCR
- RT-PCR:
-
Reverse transcriptase–polymerase chain reaction
- Suc:
-
Sucrose
References
Amiard V, Morvan-Bertrand A, Billard JP, Huault C, Keller F, Prud’homme MP (2003) Fructans, but not the sucrosyl-galactosides, raffinose and loliose, are affected by drought stress in perennial ryegrass. Plant Physiol 132:2218–2229
Asega AF, do Nascimento JRO, Schroeven L, Van den Ende W, Carvalho MAM (2008) Cloning, characterization and functional analysis of a 1-FEH cDNA from Vernonia herbacea (Vell.) Rusby. Plant Cell Physiol 49:1185–1195
Asega AF, do Nascimento TRO, Carvalho MAM (2011) Increased expression of fructan 1-exohydrolase in rhizophores of Vernonia herbacea during sprouting and exposure to low temperature. J Plant Physiol 168:558–565
Bancal P, Carpita NC, Gaudillere JP (1992) Differences in fructan accumulated in induced and field-grown wheat plants: an elongation-trimming pathway for their synthesis. New Phytol 120:313–321
Basu PS, Ali M, Chaturvedi SK (2007) Osmotic adjustment increases water uptake, remobilization of assimilates and maintains photosynthesis in chickpea under drought. Indian J Exp Biol 45:261–267
Chalmers J, Lidgett A, Cummings N, Cao Y, Forster J, Spangenberg G (2005) Molecular genetics of fructan metabolism in perennial ryegrass. Plant Biotechnol J 3:459–474
Claessens G, Van Laere A, De Proft M (1990) Purification and properties of an inulinase from chicory roots (Cichorium intybus L.). J Plant Physiol 136:35–39
Darwen CW, John P (1989) Localization of the enzymes of fructan metabolism in vacuoles isolated by a mechanical method from tubers of Jerusalem artichoke (Helianthus tuberosus L.). Plant Physiol 89:658–663
De Coninck B, Le Rov K, Francis I, Clerens S, Vergauwen R (2005) Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant, Cell Environ 28:432–443
de Mattos Paula, Arêas A, Oliveira ML, Romero Ramos CR, Sbrogio-Almeida ME, Raw I, Ho PL (2002) Synthesis of cholera toxin B subunit gene: cloning and expression of a functional 6 × His-tagged protein in Escherichia coli. Protein Expr Purif 25:481–487
De Roover J, Van Laere A, De Winter M, Van den Ende W (1999) Purification and properties of a second fructan exohydrolase from the roots of Cichorium intybus. Physiol Plant 106:28–34
De Roover J, Van den Branden K, Van Laere A, Van den Ende W (2000) Drought induces fructan synthesis and 1-SST (sucrose:sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta 210:808–814
Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129:629–636
Edelman J, Jefford T (1964) The metabolism of fructose polymers in plants. 4. β-Fructofuranosidases of tubers of Helianthus tuberosus L. Biochem J 93:148
Edelman J, Jefford T (1968) The mechanisim of fructosan metabolism in higher plants as exemplified in Helianthus tuberosus. New Phytol 67:517–531
Fu J, Huang B, Fry J (2010) Osmotic potential, sucrose level, and activity of sucrose metabolic enzymes in tall fescue in response to deficit irrigation. J Am Soc Hortic Sci 135:506–510
Gao K, Zhu T, Han G (2013) Water and nitrogen interactively increased the biomass production of Jerusalem artichoke (Helianthus tuberosus L.) in semi-arid area. Afr J Biotechnol 10:6466–6472
Goetz M, Roitsch T (1999) The different pH optima and substrate specificities of extracellular and vacuolar invertases from plants are determined by a single amino-acid substitution. Plant J 20:707–711
Guo M, Hang H, Zhu T, Zhuang Y, Chu J, Zhang S (2008) Effect of glycosylation on biochemical characterization of recombinant phytase expressed in Pichia pastoris. Enzym Microb Technol 42:340–345
Hendry GA (1993) Evolutionary origins and natural functions of fructans—a climatological, biogeographic and mechanistic appraisal. New Phytol 123:3–14
Hincha DK, Zuther E, Hellwege EM, Heyer AG (2002) Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 12:103–110
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn 347:1–32
Hu T, Hu LX, Zhang XZ, Zhang PP, Zhao ZJ, Fu JM (2013) Differential responses of CO2 assimilation, carbohydrate allocation and gene expression to NaCl stress in perennial ryegrass with different salt tolerance. PLoS ONE 8:e66090
Huang Z, Zhao L, Chen D, Liang M, Liu Z, Shao H, Long X (2013) Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 8:e62085
Ibraheem O, Dealtry G, Roux S, Bradley G (2011) The effect of drought and salinity on the expressional levels of sucrose transporters in rice (Oryza sativa Nipponbare) cultivar plants. Plant Omics 4:68–74
Ji X, Van den Ende W, Schroeven L, Clerens S, Geuten K, Cheng S, Bennett J (2007) The rice genome encodes two vacuolar invertases with fructan exohydrolase activity but lacks the related fructan biosynthesis genes of the Pooideae. New Phytol 173:50–62
Ji X, Shiran B, Wan J, Lewis DC, Jenkins CL, Condon AG, Dolferus R (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell Environ 33:926–942
Joudi M, Ahmadi A, Mohamadi V, Abbasi A, Vergauwen R, Mohammadi H, Van den Ende W (2012) Comparison of fructan dynamics in two wheat cultivars with different capacities of accumulation and remobilization under drought stress. Physiol Plant 144:1–12
Kaur S, Gupta AK, Kaur N (2003) Effect of kinetin on starch and sucrose metabolising enzymes in salt stressed chickpea seedlings. Biol Plant 46:67–72
Kawakami A, Yoshida M, Van den Ende W (2005) Molecular cloning and functional analysis of a novel 6&1-FEH from wheat (Triticum aestivum L.) preferentially degrading small graminans like bifurcose. Gene 358:93–101
Kerepesi I, Galiba G, Bányai É (1998) Osmotic and salt stresses induced differential alteration in water-soluble carbohydrate content in wheat seedlings. J Agric Food Chem 46:5347–5354
K-I Tamura, Sanada Y, Tase K, Komatsu T, Yoshida M (2011) Pp6-FEH1 encodes an enzyme for degradation of highly polymerized levan and is transcriptionally induced by defoliation in timothy (Phleum pratense L.). J Exp Bot 62:3421–3431
Kim J, Mahé A, Brangeon J, Prioul J (2000) Maize vacuolar invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol 124:71–84
Konstantinova T, Parvanova D, Atanassov A, Djilianov D (2002) Freezing tolerant tobacco, transformed to accumulate osmoprotectants. Plant Sci 163:157–164
Lammens W, Le Roy K, Schroeven L, Van Laere A, Rabijns A, Van den Ende W (2009) Structural insights into glycoside hydrolase family 32 and 68 enzymes: functional implications. J Exp Bot 60:727–740
Lasseur B, Lothier J, Djoumad A, De Coninck B, Smeekens S, Van Laere A, Van den Ende W (2006) Molecular and functional characterization of a cDNA encoding fructan:fructan 6G-fructosyltransferase (6G-FFT)/fructan:fructan 1-fructosyltransferase (1-FFT) from perennial ryegrass (Lolium perenne L.). J Exp Bot 57:2719–2734
Lasseur B, Schroeven L, Lammens W, Le Roy K, Spangenberg G, Manduzio H, Vergauwen R, Van den Ende W (2009) Transforming a fructan:fructan 6G-fructosyltransferase from perennial ryegrass into a sucrose:sucrose 1-fructosyltransferase. Plant Physiol 149:327–339
Le Roy K, Lammens W, Verhaest M, De Coninck B, Rabijns A, Van Laere A, Van den Ende W (2007a) Unraveling the difference between invertases and fructan exohydrolases: a single amino acid (Asp-239) substitution transforms Arabidopsis cell wall invertase 1 into a fructan 1-exohydrolase. Plant Physiol 145:616–625
Le Roy K, Vergauwen R, Cammaer V, Yoshida M, Kawakami A, Van Laere A, Van den Ende W (2007b) Fructan 1-exohydrolase is associated with flower opening in Campanula rapunculoides. Funct Plant Biol 34:972–983
Li H, Xu H, Zhao G, Liang M (2014) The effect of deformation on dry matter and sugar accumulation and their distribution profiles in Jerusalem artichoke. Acta Pratacult Sin 23:149–157
Liang M, Hole D, Wu J, Blake T, Wu Y (2012) Expression and functional analysis of NUCLEAR FACTOR-Y, subunit B genes in barley. Planta 235:779–791
Liang M, Yin X, Lin Z, Zheng Q, Liu G, Zhao G (2014) Identification and characterization of NF-Y transcription factor families in Canola (Brassica napus L.). Planta 239:107–126
Liu D, Li J, Zhao S, Zhang R, Wang M, Miao Y, Shen Y (2013) Secretome diversity and quantitative analysis of cellulolytic Aspergillus fumigatus Z5 in the presence of different carbon sources. Biotechnol Biofuels 6:149
Livingston DP, Henson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol 116:403–408
Livingston DP III, Hincha DK, Heyer AG (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66:2007–2023
Lothier J, Lasseur B, Le Roy K, Van Laere A, Barre P, Van den Ende W (2007) Cloning, gene mapping, and functional analysis of a fructan 1-exohydrolase (1-FEH) from Lolium perenne implicated in fructan synthesis rather than in fructan mobilization. J Exp Bot 58:1969–1983
Marx SP, Nosberger J, Frehner M (1997) Seasonal variation of fructan-β-fructosidase (FEH) activity and characterization of a β-(2-1)-linkage specific FEH from tubers of Jerusalem artichoke (Helianthus tuberosus). New Phytol 135:267–277
Nguyen G, Hailstones D, Wilkes M, Sutton B (2010) DROUGHT STRESS: role of carbohydrate metabolism in drought-induced male sterility in rice anthers. J Agron Crop Sci 196:346–357
Oliveira V, Silva E, Zaidan L, Carvalho M (2013) Effects of elevated CO2 concentration and water deficit on fructan metabolism in Viguiera discolor Baker. Plant Biol 15:471–482
Pilon-Smits EA, Ebskamp MJ, Paul MJ, Jeuken MJ, Weisbeek PJ, Smeekens SC (1995) Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol 107:125–130
Pollock C, Farrar J, Tomos D, Gallagher J, Lu C, Koroleva O (2003) Balancing supply and demand: the spatial regulation of carbon metabolism in grass and cereal leaves. J Exp Bot 54:489–494
Pommerrenig B, Papini-Terzi FS, Sauer N (2007) Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiol 144:1029–1038
Resina D, Ac Serrano, Valero F, Ferrer P (2004) Expression of a Rhizopus oryzae lipase in Pichia pastoris under control of the nitrogen source-regulated formaldehyde dehydrogenase promoter. J Biotechnol 109:103–113
Ritsema T, Smeekens S (2003a) Fructans: beneficial for plants and humans. Curr Opin Plant Biol 6:223–230
Ritsema T, Smeekens S (2003b) Engineering fructan metabolism in plants. J Plant Physiol 160:811–820
Rosa M, Hilal M, Gonzalez JA, Prado FE (2009) Low-temperature effect on enzyme activities involved in sucrose-starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiol Biochem 47:300–307
Schroeven L, Lammens W, Van Laere A, Van den Ende W (2008) Transforming wheat vacuolar invertase into a high affinity sucrose: sucrose 1-fructosyltransferase. New Phytol 180:822–831
Spollen WG, Nelson CJ (1994) Response of fructan to water deficit in growing leaves of tall fescue. Plant Physiol 106:329–336
Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A (1995) Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci 92:11652–11656
Trouverie J, Thevenot C, Rocher J, Sotta B, Prioul J (2003) The role of abscisic acid in the response of a specific vacuolar invertase to water stress in the adult maize leaf. J Exp Bot 54:2177–2186
Ueno K, Ishiguro Y, Yoshida M, Onodera S, Shiomi N (2011) Cloning and functional characterization of a fructan 1-exohydrolase (1-FEH) in edible burdock (Arctium lappa L.). Chem Cent J 5:1–9
Valluru R, Van den Ende W (2008) Plant fructans in stress environments: emerging concepts and future prospects. J Exp Bot 59:2905–2916
Van den Ende W, Michiels A, De Roover J, Verhaert P, Van Laere A (2000) Cloning and functional analysis of chicory root fructan 1-exohydrolase I (1-FEH I): a vacuolar enzyme derivedfrom a cell-wall invertase ancestor? Mass fingerprint of the 1-FEH I enzyme. Plant J 24:447–456
Van den Ende W, Michiels A, Van Wonterghem D, Clerens SP, De Roover J, Van Laere AJ (2001) Defoliation induces fructan 1-exohydrolase II in witloof chicory roots. Cloning and purification of two isoforms, fructan 1-exohydrolase IIa and fructan 1-exohydrolase IIb. Mass fingerprint of the fructan 1-exohydrolase II enzymes. Plant Physiol 126:1186–1195
Van den Ende W, Clerens S, Vergauwen R, Van Riet L, Van Laere A, Yoshida M, Kawakami A (2003a) Fructan 1-exohydrolases. β-(2, 1)-trimmers during graminan biosynthesis in stems of wheat? Purification, characterization, mass mapping, and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 131:621–631
Van den Ende W, De Coninck B, Clerens S, Vergauwen R, Van Laere A (2003b) Unexpected presence of fructan 6-exohydrolases (6-FEHs) in non-fructan plants: characterization, cloning, mass mapping and functional analysis of a novel ‘cell-wall invertase-like’ specific 6-FEH from sugar beet (Beta vulgaris L.). Plant J 36:697–710
Van den Ende W, De Coninck B, Van Laere A (2004) Plant fructan exohydrolases: a role in signaling and defense? Trends Plant Sci 9:523–528
Van den Ende W, Yoshida M, Clerens S, Vergauwen R, Kawakami A (2005) Cloning, characterization and functional analysis of novel 6-kestose exohydrolases (6-KEHs) from wheat (Triticum aestivum). New Phytol 166:917–932
Van den Ende W, Clerens S, Vergauwen R, Boogaerts D, Le Roy K, Arckens L, Van Laere A (2006) Cloning and functional analysis of a high DP fructan:fructan 1-fructosyl transferase from Echinops ritro (Asteraceae): comparison of the native and recombinant enzymes. J Exp Bot 57:775–789
Van den Ende W, Lammens W, Van Laere A, Schroeven L, Le Roy K (2009) Donor and acceptor substrate selectivity among plant glycoside hydrolase family 32 enzymes. FEBS J 276:5788–5798
Van Der Meer IM, Koops AJ, Hakkert JC, Van Tunen AJ (1998) Cloning of the fructan biosynthesis pathway of Jerusalem artichoke. Plant J 15:489–500
Van Laere A, Van den Ende W (2002) Inulin metabolism in dicots: chicory as a model system. Plant, Cell Environ 25:803–813
Vandoorne B, Mathieu A-S, Van den Ende W, Vergauwen R, Périlleux C, Javaux M, Lutts S (2012) Water stress drastically reduces root growth and inulin yield in Cichorium intybus (var. sativum) independently of photosynthesis. J Exp Bot 63:4359–4373
Verhaest M, Van den Ende W, Roy KL, De Ranter CJ, Van Laere A, Rabijns A (2005) X-ray diffraction structure of a plant glycosyl hydrolase family 32 protein: fructan 1-exohydrolase IIa of Cichorium intybus. Plant J 41:400–411
Verhaest M, Lammens W, Le Roy K, De Ranter CJ, Van Laere A, Rabijns A, Van den Ende W (2007) Insights into the fine architecture of the active site of chicory fructan 1-exohydrolase: 1-kestose as substrate vs sucrose as inhibitor. New Phytol 174:90–100
Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120:351–360
Wagner W, Wiemken A (1986) Properties and subcellular localization of fructan hydrolase in the leaves of barley (Hordeum vulgare L. cv Gerbel). J Plant Physiol 123:429–439
Wang L, Huang Y, Long X, Meng X, Liu Z (2011) Cloning of exoinulinase gene from Penicillium janthinellum strain B01 and its high-level expression in Pichia pastoris. J Appl Microbiol 111:1371–1380
Zhang J, Dell B, Conocono E, Waters I, Setter T, Appels R (2009) Water deficits in wheat: fructan exohydrolase (1-FEH) mRNA expression and relationship to soluble carbohydrate concentrations in two varieties. New Phytol 181:843–850
Zhao G, Liu Z, Chen M, Kou W (2006) Effect of saline aquaculture effluent on salt-tolerant Jerusalem Artichoke (Helianthus tuberosus L.) in a semi-arid coastal area of China. Pedosphere 16:762–769
Zhao G, Liu Z, Chen M (2008) Soil properties and yield of Jerusalem artichoke (Helianthus tuberosus L.) with seawater irrigation in North China Plain. Pedosphere 18:195–202
Acknowledgments
We thank Kathleen Farquharson for valuable comments on the manuscript revision. This research was supported by grants from the National Natural Science Foundation of China (31201842), the National High Technology Research and Development Program (“863”Program, 2011AA100209), the Doctoral Program of Higher Education of China (20120097120015), Fundamental Research Funds for the Central Universities (KYZ201206), the Priority Academic Program Development of Jiangsu Higher Education Institutions (RAPD program, 809001), and the Scientific Research Foundation of the State Human Resource Ministry.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, H., Liang, M., Xu, L. et al. Cloning and functional characterization of two abiotic stress-responsive Jerusalem artichoke (Helianthus tuberosus) fructan 1-exohydrolases (1-FEHs). Plant Mol Biol 87, 81–98 (2015). https://doi.org/10.1007/s11103-014-0262-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-014-0262-1