ABSTRACT
Purpose
To enhance efficacy, bioavailability and reduce toxicity of first-line highly active anti-retroviral regimen, zidovudine + efavirenz + lamivudine loaded lactoferrin nanoparticles were prepared (FLART-NP) and characterized for physicochemical properties, bioactivity and pharmacokinetic profile.
Methods
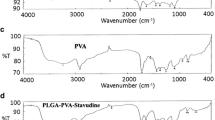
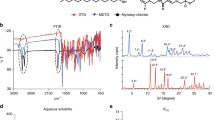
Nanoparticles were prepared using sol-oil protocol and characterized using different sources such as FE-SEM, AFM, NanoSight, and FT-IR. In-vitro and in-vivo studies have been done to access the encapsulation-efficiency, cellular localization, release kinetics, safety analysis, biodistribution and pharmacokinetics.
Results
FLART-NP with a mean diameter of 67 nm (FE-SEM) and an encapsulation efficiency of >58% for each drug were prepared. In-vitro studies suggest that FLART-NP deliver the maximum of its payload at pH5 with a minimum burst release throughout the study period with negligible toxicity to the erythrocytes plus improved in-vitro anti-HIV activity. FLART-NP has improved the in-vivo pharmacokinetics (PK) profiles over the free drugs; an average of >4fold increase in AUC and AUMC, 30% increase in the Cmax, >2fold in the half-life of each drug. Biodistribution data suggest that FLART-NP has improved the bioavailability of all drugs with less tissue-related inflammation as suggested with histopathological evaluation
Conclusions
The triple-drug loaded nanoparticles have various advantages against soluble (free) drug combination in terms of enhanced bioavailability, improved PK profile and diminished drug-associated toxicity.







Similar content being viewed by others
Abbreviations
- 3TC or LMV:
-
Lamivudine
- ART:
-
Antiretroviral therapy
- ARV:
-
Antiretroviral
- AZT:
-
Azidothymidine or Zidovudine
- DL:
-
Drug loading
- DMSO:
-
Di methyl sulfoxide
- EE:
-
Encapsulation efficiency
- EFV:
-
Efavirenz
- FLART-NP:
-
First-Line ART Nanoparticles
- FT-IR:
-
Fourier transform infrared spectroscopy
- HPLC:
-
High Performance Liquid Chromatography
- HR:
-
Hemolysis rate
- IC50 :
-
50% Inhibitory concentration
- Lf:
-
Lactoferrin
- NP:
-
Nanoparticles
- NTA:
-
Nanoparticle tracking analysis
- PBS:
-
Phosphate-buffered saline
- sol:
-
Soluble or free
REFERENCES
Langeand JM, Schwartlander B. Introduction 15 million on ART by 2015: a realistic target or just a dream. Curr Opin HIV AIDS. 2013;8:1–3.
Artsand EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2:a007161.
Boyapalle S, Mohapatra S, Mohapatra S. Nanotechnology applications to HIV vaccines and microbicides. J Global Infect Dis. 2012;4:62–8.
Nittayananta W, Talungchit S, Jaruratanasirikul S, Silpapojakul K, Chayakul P, Nilmanat A, et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med: Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2010;39:397–406.
Younai FS. Thirty years of the human immunodeficiency virus epidemic and beyond. Int J Oral Sci. 2013;5:191–9.
Perno CF. The discovery and development of HIV therapy: the new challenges. Ann Ist Super Sanita. 2011;47:41–3.
E.H. Humphreys, L.W. Chang, and J. Harris. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev. CD006517 (2010).
Kebba A, Atwine D, Mwebaze R, Kityo C, Nakityo R, Peter M. Therapeutic responses to AZT + 3TC + EFV in advanced antiretroviral naive HIV type 1-infected Ugandan patients. AIDS Res Hum Retrovir. 2002;18:1181–7.
Trotta MP, Ammassari A, Melzi S, Zaccarelli M, Ladisa N, Sighinolfi L, et al. Treatment-related factors and highly active antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2002;31 Suppl 3:S128–131.
Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem. 2014;53:12320–64.
Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retrovir. 2015;31:107–14.
Khalil NM, Carraro E, Cotica LF, Mainardes RM. Potential of polymeric nanoparticles in AIDS treatment and prevention. Expert Opin Drug Deliv. 2011;8:95–112.
Leyva-Gomez G, Cortes H, Magana JJ, Leyva-Garcia N, Quintanar-Guerrero D, Floran B. Nanoparticle technology for treatment of Parkinson’s disease: the role of surface phenomena in reaching the brain. Drug Discov Today. 2015;20:824–37.
Panyamand J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47.
Jacobson GB, Shinde R, Contag CH, Zare RN. Sustained release of drugs dispersed in polymer nanoparticles. Angew Chem. 2008;47:7880–2.
Jia L. Nanoparticle formulation increases oral bioavailability of poorly soluble drugs: approaches experimental evidences and theory. Curr Nanosci. 2005;1:237–43.
Cho M, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, et al. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol Lett. 2009;189:177–83.
Florisa R, Recio I, Berkhout B, Visser S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr Pharm Des. 2003;9:1257–75.
Leon-Sicairos N, Reyes-Lopez M, Ordaz-Pichardo C, de la Garza M. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem Cell Biol. 2006;84:327–36.
Ahmed F, Ali MJ, Kondapi AK. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int J Biol Macromol. 2014;70:572–82.
Kumar P, Lakshmi YS, Golla BCK, Kondapi AK. Improved safety, bioavailability and pharmacokinetics of zidovudine through lactoferrin nanoparticles during oral administration in rats. PLoS ONE. 2015;10:e0140399.
Krishna AD, Mandraju RK, Kishore G, Kondapi AK. An efficient targeted drug delivery through apotransferrin loaded nanoparticles. PLoS ONE. 2009;4:e7240.
Kalaivani T, Rajasekaran C, Suthindhiran K, Mathew L. Free radical scavenging, cytotoxic and hemolytic activities from leaves of acacia nilotica (L.) wild. ex. Delile subsp. Indica (Benth.) Brenan. Evid Based Complement Alternat Med: eCAM. 2011;2011, 274741.
Zhang J, Chen XG, Li YY, Liu CS. Self-assembled nanoparticles based on hydrophobically modified chitosan as carriers for doxorubicin. Nanomed: Nanotechnol Biol Med. 2007;3:258–65.
Burger DM, Rosing H, Koopman FJ, Mennhorst PL, Mulder JW, Bult A, et al. Determination of 3′-amino-3′-deoxythymidine, a cytotoxic metabolite of 3′-azido-3′-deoxythymidine, in human plasma by ion-pair high-performance liquid chromatography. J Chromatogr. 1993;622:235–42.
Bienvenu E, Hoffmann KJ, Ashton M, Kayumba PC. A rapid and selective HPLC-UV method for the quantitation of efavirenz in plasma from patients on concurrent HIV/AIDS and tuberculosis treatments. Biomed Chromatogr: BMC. 2013;27:1554–9.
Alebouyeh M, Amini H. Rapid determination of lamivudine in human plasma by high-performance liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci. 2015;975:40–4.
Wang SB, Chen AZ, Weng LJ, Chen MY, Xie XL. Effect of drug-loading methods on drug load, encapsulation efficiency and release properties of alginate/poly-L-arginine/chitosan ternary complex microcapsules. Macromol Biosci. 2004;4:27–30.
Golla K, Cherukuvada B, Ahmed F. Kondapi AKEfficacy, safety and anticancer activity of protein nanoparticle-based delivery of doxorubicin through intravenous administration in rats. PLoS One. 2012;7(12), e51960.
Kim MJ, Shin S. Toxic effects of silver nanoparticles and nanowires on erythrocyte rheology. Food Chem Toxicol. 2014;67:80–6.
T Mocan. Hemolysis as expression of nanoparticles - induced cytotoxicity in red blood cells. 2013;1:7–12.
Ciaffi L, Koulla-Shiro S, Sawadogo A, le Moing V, Eymard-Duvernay S, Izard S, et al. Efficacy and safety of three second-line antiretroviral regimens in HIV-infected patients in Africa. AIDS. 2015;29:1473–81.
Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70.
3rd Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102.
Gaur PK, Mishra S, Bajpai M, Mishra A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: in-vitro drug release and pharmacokinetics studies. Biomed Res Int. 2014;2014:363404.
Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349–58.
Abd Elgadir M, Uddin S, Ferdosh S, Adam A, Chowdhury AJK, Sarker ZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J Food Drug Anal. 2014;23:619–29.
Koduriand PR, Parekh S. Zidovudine-related anemia with reticulocytosis. Ann Hematol. 2003;82:184–5.
ACKNOWLEDGMENTS AND DISCLOSURES
PK (orcid.org/0000-0003-1038-6149) is UGC-NET fellow, YSL is ICMR-SRF fellow. This work was supported by Department of Science and Technology under Nano mission (#SR/NM/NS-1127/2011). AKK is recipient of FRPS BSR UGC one time grant. PK and AKK conceived and designed the experiments. PK and YSL did the experiments and analyzed the data. AKK and PK wrote the manuscript. The authors report no conflicts of interest in this work
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 489 kb)
Rights and permissions
About this article
Cite this article
Kumar, P., Lakshmi, Y.S. & Kondapi, A.K. Triple Drug Combination of Zidovudine, Efavirenz and Lamivudine Loaded Lactoferrin Nanoparticles: an Effective Nano First-Line Regimen for HIV Therapy. Pharm Res 34, 257–268 (2017). https://doi.org/10.1007/s11095-016-2048-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2048-4