Abstract
Purpose
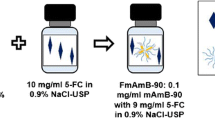
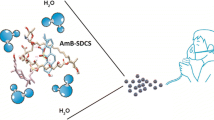
Fungizone® (AmB-SD), amphotericin B solubilized by sodium deoxycholate, contains a highly aggregated form of the antifungal agent that causes dose-limiting renal toxicity. With the aim of reducing the formulation’s toxicity by co-delivering monomeric amphotericin B (AmB) and sodium supplementation, we deaggregated AmB-SD with FDA-approved excipient PEG-DSPE in 0.9% NaCl-USP. Herein, we describe a reformulated AmB-SD with PEG-DSPE micelles that results in a less toxic drug with maintained antifungal activity.
Methods
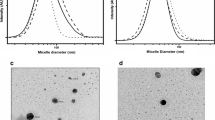
We compared the aggregation state and particle size of AmB-SD alone or combined with PEG-DSPE micelles. In vitro hemolytic activity and in vivo renal toxicity were measured to determine the toxicity of different formulations. In vitro antifungal assays were performed to determine differences in efficacy among formulations.
Results
PEG-DSPE micelles in saline deaggregated AmB-SD. Deaggregated AmB-SD exhibited significantly reduced in vitro and in vivo toxicity. In vitro antifungal studies showed no difference in minimum inhibitory and fungicidal concentrations of AmB-SD combined with PEG-DSPE relative to the drug alone.
Conclusions
Reformulation of AmB-SD with PEG-DSPE micelles in saline facilitates co-delivery of monomeric AmB and sodium supplementation, potentially reducing the dose-limiting nephrotoxicity of AmB-SD. Ease of preparation and commercially available components lead us to acknowledge its potential for clinical use.






Similar content being viewed by others
Abbreviations
- AmB:
-
Amphotericin B
- AmB-SD:
-
Fungizone
- BUN:
-
Blood urea nitrogen
- CAC:
-
Critical aggregation concentration
- MFC:
-
Minimum fungicidal concentration
- MIC:
-
Minimum inhibitory concentration
- SD:
-
Sodium deoxycholate
- SDA:
-
Sabouraud dextrose agar
- TL:
-
Total lysis
- YPD:
-
Yeast peptone dextrose
References
Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A. 2012;109(7):2234–9.
Barwicz J, Christian S, Gruda I. Effects of the aggregation state of amphotericin-b on its toxicity to mice. Antimicrob Agents Chemother. 1992;36(10):2310–5.
Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: time for a new “gold standard”. Clin Infect Dis. 2003;37(3):415–25.
Cleary JD, Rogers PD, Chapman SW. Variability in polyene content and cellular toxicity among deoxycholate amphotericin B formulations. Pharmacotherapy. 2003;23(5):572–8.
Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DRJ, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001;32(5):686–93.
Lamyfreund MT, Schreier S, Peitzsch RM, Reed WF. Characterization and time-dependence of amphotericin-B - deoxycholate aggregation by quasi-elastic light-scattering. J Pharm Sci. 1991;80(3):262–6.
Inselmann G, Balaschke M, Heidemann HT. Enzymuria following amphotericin B application in the rat. Mycoses. 2003;46(5–6):169–73.
Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. Amphotericin B formulations and drug targeting. J Pharm Sci. 2008;97(7):2405–25.
Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46(3):828–33.
Cagnoni PJ, Walsh TJ, Prendergast MM, Bodensteiner D, Hiemenz S, Greenberg RN, et al. Pharmacoeconomic analysis of liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients. J Clin Oncol. 2000;18(12):2476–83.
Falci DR, da Rosa FB, Pasqualotto AC. Comparison of nephrotoxicity associated to different lipid formulations of amphotericin B: a real-life study. Mycoses. 2015;58(2):104–12.
Espuelas MS, Legrand P, Cheron M, Barratt G, Puisieux F, Devissaguet JP, et al. Interaction of amphotericin B with polymeric colloids - A spectroscopic study. Colloids Surf B Biointerfaces. 1998;11(3):141–51.
Kajtar M, Vikmon M, Morlin E, Szejtli J. Aggregation of amphotericin-b in the presence of gamma-cyclodextrin. Biopolymers. 1989;28(9):1585–96.
Tancrede P, Barwicz J, Jutras S, Gruda I. The effect of surfactants on the aggregation state of amphotericin-B. Biochim Biophys Acta. 1990;1030(2):289–95.
Gruda I, Dussault N. Effect of the aggregation state of amphotericin-b on its interaction with ergosterol. Biochem Cell Biol-Biochimie Et Biologie Cellulaire. 1988;66(3):177–83.
Gruda I, Gauthier E, Elberg S, Brajtburg J, Medoff G. Effects of the detergent sucrose monolaurate on binding of amphotericin-B to sterols and its toxicity for cells. Biochem Biophys Res Commun. 1988;154(3):954–8.
Gruda I, Milette D, Brother M, Kobayashi GS, Medoff G, Brajtburg J. Structure-activity study of inhibition of amphotericin-B (Fungizone) binding to sterols, toxicity to cells, and lethality to mice by esters of sucrose. Antimicrob Agents Chemother. 1991;35(1):24–8.
Bolard J, Legrand P, Heitz F, Cybulska B. One-sided action of amphotericin-B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991;30(23):5707–15.
Legrand P, Romero EA, Cohen BE, Bolard J. Effects of aggregation and solvent on the toxicity of amphotericin-B to human erythrocytes. Antimicrob Agents Chemother. 1992;36(11):2518–22.
Branch RA. Prevention of amphotericin-B induced renal impairment - a review on the use of sodium supplementation. Arch Intern Med. 1988;148(11):2389–94.
Llanos A, Cieza J, Bernardo J, Echevarria J, Biaggioni I, Sabra R, et al. Effect of salt supplementation on amphotericin-b nephrotoxicity. Kidney Int. 1991;40(2):302–8.
Ohnishi A, Ohnishi T, Stevenhead W, Robinson RD, Glick A, Oday DM, et al. Sodium status influences chronic amphotericin-b nephrotoxicity in rats. Antimicrob Agents Chemother. 1989;33(8):1222–7.
Mayer J, Doubek M, Doubek J, Horky D, Scheer P, Stepanek M. Reduced nephrotoxicity of conventional amphotericin B therapy after minimal nephroprotective measures: animal experiments and clinical study. J Infect Dis. 2002;186(3):379–88.
Sawaya BP, Briggs JP, Schnermann J. Amphotericin-B nephrotoxicity - the adverse consequences of altered membrane-properties. J Am Soc Nephrol. 1995;6(2):154–64.
Carlson MA, Condon RE. Nephrotoxicity of amphotericin-B. J Am Coll Surg. 1994;179(3):361–81.
Adams ML, Andes DR, Kwon GS. Amphotericin B encapsulated in micelles based on poly(ethylene oxide)-block-poly(L-amino acid) derivatives exerts reduced in vitro hemolysis but maintains potent in vivo antifungal activity. Biomacromolecules. 2003;4(3):750–7.
Diezi TA, Kwon G. Amphotericin B/sterol co-loaded PEG-phospholipid micelles: effects of sterols on aggregation state and hemolytic activity of amphotericin B. Pharm Res. 2012;29(7):1737–44.
Yu BG, Okano T, Kataoka K, Sardari S, Kwon GS. In vitro dissociation of antifungal efficacy and toxicity for amphotericin B-loaded poly(ethylene oxide)-block-poly( beta-benzyl-L-aspartate) micelles. J Control Release. 1998;56(1–3):285–91.
Lavasanifar A, Samuel J, Sattari S, Kwon GS. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharm Res. 2002;19(4):418–22.
Diezi TA, Takemoto JK, Davies NM, Kwon GS. Pharmacokinetics and nephrotoxicity of amphotericin B-incorporated poly(ethylene glycol)-block-poly(N-hexyl stearate l-aspartamide) micelles. J Pharm Sci. 2011;100(6):2064–70.
Bolard J, Seigneuret M, Boudet G. Interaction between phospholipid-bilayer membranes and the polyene antibiotic amphotericin-b - lipid state and cholesterol content dependence. Biochim Biophys Acta. 1980;599(1):280–93.
Aramwit P, Yu BG, Lavasanifar A, Samuel J, Kwon GS. The effect of serum albumin on the aggregation state and toxicity of amphotericin B. J Pharm Sci. 2000;89(12):1589–93.
Johnson EM, Ojwang JO, Szekely A, Wallace TL, Warnock DW. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother. 1998;42(6):1412–6.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the NIH (R01 AI-43346). Special thanks to Dr. Tim Bugni for kindly providing C. albicans strain K1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez, C., Shin, D.H. & Kwon, G.S. Reformulation of Fungizone by PEG-DSPE Micelles: Deaggregation and Detoxification of Amphotericin B. Pharm Res 33, 2098–2106 (2016). https://doi.org/10.1007/s11095-016-1948-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1948-7