Abstract
Purpose
The effectiveness of Tenofovir based HIV pre-exposure prophylaxis (PrEP) is proven, but hinges on correct and consistent use. User compliance and therapeutic effectiveness can be improved by long acting drug delivery systems. Here we describe a thin-film polymer device (TFPD) as a biodegradable subcutaneous implant for PrEP.
Methods
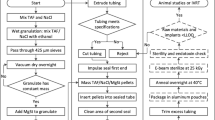
A thin-film polycaprolactone (PCL) membrane controls drug release from a reservoir. To achieve membrane controlled release, TAF requires a formulation excipient such as PEG300 to increase the dissolution rate and reservoir solubility. Short-term In vitro release studies are used to develop an empirical design model, which is applied to the production of in vitro prototype devices demonstrating up to 90-days of linear release and TAF chemical stability.
Results
The size and shape of the TFPD are tunable, achieving release rates ranging from 0.5 to 4.4 mg/day in devices no larger than a contraceptive implant. Based on published data for oral TAF, subcutaneous constant-rate release for HIV PrEP is estimated at <2.8 mg/day. Prototype devices demonstrated linear release at 1.2 mg/day for up to 90 days and at 2.2 mg/day for up to 60 days.
Conclusions
We present a biodegradable TFPD for subcutaneous delivery of TAF for HIV PrEP. The size, shape and release rate of the device are tunable over a >8-fold range.






Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- ARV:
-
Antiretroviral
- DC:
-
Direct current
- FDC:
-
Emtricitabine
- HPLC:
-
High performance liquid chromatography
- LA-PrEP:
-
Long acting pre-exposure prophylaxis
- PCL:
-
Polycaprolactone
- PDMS:
-
Polydimethylsiloxane
- PEG:
-
Polyethylene glycol
- PrEP:
-
Pre-exposure prophylaxis
- TAF:
-
Tenofovir alafenamide fumarate
- TDF:
-
Tenofovir disproxil fumarate
- TFPD:
-
Thin film Polycaprolactone device
- TFV:
-
Tenofovir
- UV:
-
Ultraviolet
References
Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral preexposure prophylaxis. Annu Rev Med. 2013;64:219–32.
McGowan I. An overview of antiretroviral pre-exposure prophylaxis of HIV infection. Am J Reprod Immunol. 2014;71(6):624–30.
Amico K, Mansoor L, Corneli A, Torjesen K, van der Straten A. Adherence support approaches in biomedical HIV prevention trials: experienes, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17:2143–55.
N. I. o. A. a. I. D. (NIAID), “Emtricitabine/Tenofovir Disproxil Fumarate for HIV Prevention in Men (iPrEx),” clinicaltrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US), 2000-[cited 2015 Aug 02].
U. o. Washington, “Pre-Exposure Prophylaxis to Prevent HIV-1 Acquisition Within HIV-1 Discordant Couples (Partners PrEP),” Clinicaltrials.gov [internet]. Bethesda (MD): National Library of Medicine (US)., 2000-[cited 2015 Aug 02].
Marrazzo J, Ramjee G, Richardson B, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:50–518.
Van Damme L, Corneli A, Ahemd K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22.
N. I. o. A. a. I. D. NIAID, “Safety and Effeciveness of Tenofovir 1% Gel, Tenofovir Disproxil Fumarate, and Emtricitabine/Tenofovir Disproxil Fumarate Tablets in Preventing HIV in Women,” ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US)., 2000-[cited 2015 Aug 02].
Spreen W, Margolis D, Pottage J. Long-acting antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565–71.
van’t Klooster G, Hoeben E, Borghys H, Looszova A, Bouche M, van Velsen F, et al. Pharmacokinetics and Disoposition of Rilpivirine (TMC278) Nanosuspension as a Long-acting Injectable Antiretroviral Formulation. Antimicrob Agents Chemother. 2010;54(5):2042–50.
Spreen W, Ford S, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 Pharmacokinetics in Plasma and Tissue After Single-Dose Long Acting Injectable Administration in Healthy Subjects. J Acquir Immun Defic Syndr. 2014;67(5):481–6.
Taylor S, Boffito M, Khoo S, Smit E, Back D. Stopping antiretroviral therapy. AIDS. 2007;21:1673–82.
Lee W, He G, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, et al. Selective Intracellular Activation of a Novel Prodrug of the Human Immunodeficiency Virus Reverse Transcriptase Inhibitor Tenofovir Leads to Preferential Distribution and Accumulation in Lymphatic Tissue. Antimicrob Agents Chemother. 2005;49(5):1989–06.
Falutz J. Unmasking the bare bones of HIV Preexposure Prophylaxis. Clin Infect Dis. 2015;1–3.
Grant R, Lama J, Anderson P, McMahan V, Liu A, Vargas L, et al. Preexposure prophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99.
U. o. N. C. C. H. (NCT02357602), “Dose Proportionality of TFV-DP After a Single Dose of GS-7340 in Women,” ClinicalTrials.gov, 2015.
Ruane P et al. Antiviral Activity and Safety, and Pharmacokinetics/Pharmacodynamics of Tenofovir Alafenamide as 10-day Monotherapy in HIV-1 Positive Adults. J Acquir Immune Defic Syndr. 2013;63(4):449–55.
Babusis D et al. Mechanism for Effective Lymphoid Cell and Tissue Loading Following Oral Administration fo Nucleotide Prodrug GS-7340. Mol Pharm. 2013;10(2):459–66.
Gunawardana M, Remedios-Chan M, Miller C, Fanter R, Yang F, Marzinke M, et al. Pharmacokinetics of Long-Acting Tenofovir Alafenimide (GS-7340) Subdermapl Implant for HIV Prophylaxis. Antimicrob Agents Chemother. 2015;59(7):3913–9.
Siepmann J, Siepmann F. Modeling of diffusion controlled drug delivery. J Control Release. 2012;161:351–62.
Schlesinger E, Ciaccio N, Desai T. Polycaprolactone Thin-Film Drug Delivery Systems: Empirical and Predictive Models for Device Design. Mater Sci Eng C Mater Biol Appl. 2015;57:232–9.
Lance K, Goods S, Mendes T, Ishikiriyama M, Chew P, Estes L, et al. In vitor and in vivo sustained zero-order delivery of Rapamycin (Sirolimus) from a biodegradable intraocular device. Invest Opthalmol Vis Sci. 2015;56:7331–7.
Sun H, Mei L, Song C, Cui X, Wang P. The in vivo Degradation, Absoprtion, and Excetion of PCL-based Implant. Biomaterials. 2006;27:1735–40.
Woodruff M, Hutmacher D. The Return of the Forgotten Polymer: Polycaprolactone in the 21st Century. Prog Polym Sci. 2010;35(10):1217–56.
Acknowledgments and Disclosures
This research is made possible by the generous support of the American people through the U.S. President’s Emergency Plan for AIDS Relief. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, PEPFAR, or the United States Government.
Tenofovir Alafeinimde Fumarate drug substance and analytical methods were graciously provided by Gilead Sciences, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlesinger, E., Johengen, D., Luecke, E. et al. A Tunable, Biodegradable, Thin-Film Polymer Device as a Long-Acting Implant Delivering Tenofovir Alafenamide Fumarate for HIV Pre-exposure Prophylaxis. Pharm Res 33, 1649–1656 (2016). https://doi.org/10.1007/s11095-016-1904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1904-6