Abstract
Purpose
The purpose of this study was to evaluate the specifically targeted efficiency of budesonide loaded PLGA nanoparticles for the treatment of inflammatory bowel disease (IBD).
Methods
The nanoparticles were prepared by an oil/water (O/W) emulsion evaporation technique. The nanoparticles were characterized for their size, shape and in vitro drug release profile. Solid state characterization was carried out by differential scanning calorimetry (DSC) and X-ray Power diffraction (XPRD). In order to evaluate the targeted efficiency of nanoparticles, a particle localization study in the healthy and in the inflamed colon was determined in vivo. These data were complemented by cryo-sections.
Results
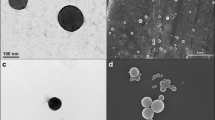
Nanoparticles were 200 ± 05 nm in size with a smooth and spherical shape. The encapsulation efficiency was around 85 ± 3.5%, which was find-out by both, direct and indirect methods. Release of budesonide from the nanoparticles showed a biphasic release profile with an initial burst followed by sustained release. XPRD data revealed that the drug in the polymer matrix existed in crystalline state. Nanoparticles accumulation in inflamed tissues was evaluated by in-vivo imaging system and it was found that particles are accumulated in abundance at the site of inflammation when compared to the healthy group.
Conclusion
The study demonstrates that the budesonide loaded PLGA nanoparticles are an efficient delivery system for targeted drug delivery to the inflamed intestinal mucosa.






Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- CD:
-
Crohn’s disease
- DSC:
-
Differential scanning calorimetry
- GIT:
-
Gastrointestinal tract
- IBD:
-
Inflammatory bowel disease
- O/W:
-
Oil/water
- PBS:
-
Phosphate buffer saline
- PDI:
-
Poly-dispersity index
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PVA:
-
Poly-vinyl alcohol
- SD:
-
Standard deviation
- SEM:
-
Scanning electron microscope
- UC:
-
Ulcerative colitis
- XRD:
-
X-ray diffraction
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–29.
Head KA, Jurenka JS. Inflammatory bowel disease part 1: ulcerative colitis—pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8(3):247–83.
Shanahan F. Crohn’s disease. Lancet. 2002;359(9300):62–9.
Gramlich T, Petras RE. Pathology of inflammatory bowel disease. Semin Pediatr Surg. 2007;16(3):154–63.
Koutroubakis I, Manousos ON, Meuwissen SG, Pena AS. Environmental risk factors in inflammatory bowel disease. Hepatogastroenterology. 1996;43(8):381–93.
Nguyen GC, Torres EA, Regueiro M, Bromfield G, Bitton A, Stempak J, et al. Inflammatory bowel disease characteristics among African Americans, hispanics, and non-Hispanic Whites: characterization of a large north American cohort. Am J Gastroenterol. 2006;101:1012–23.
Fiorino G, Fries W, De La Rue SA, Malesci AC, Repici A, Danese S. New drug delivery systems in inflammatory bowel disease: MMX and tailored delivery to the gut. Curr Med Chem. 2010;17(17):1851–7.
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–57.
Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci. 2003;44:192–201.
Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson ABR, et al. Oral budesonide for active Crohns-disease. N Engl J Med. 1994;331:836–41.
Lofberg R, Rutgeerts P, Malchow H, Lamers C, Danielsson A, Olaison G, et al. Budesonide prolongs time to relapse in ileal and ileocaecal Crohn’s disease. A placebo controlled one year study. Gut. 1996;39:82–6.
Hanauer SB. New steroids for IBD: progress report. Gut. 2002;51:182–3.
Meissner Y, Lamprecht A. Alternative drug delivery approaches for the therapy of inflammatory bowel disease. J Pharm Sci. 2008;97(8):2878–91.
Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Maincent P, et al. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2001;299(2):775–81.
Lamprecht A. IBD: selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol Hepatol. 2010;7(6):311–2.
Nakase H, Okazaki K, Tabata Y, Uose S, Ohana M, Uchida K, et al. Development of an oral drug delivery system targeting immune-regulating cells in experimental inflammatory bowel disease: a new therapeutic strategy. J Pharmacol Exp Ther. 2000;292:15–21.
Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. A pH-sensitive microsphere system for the colon delivery of tacrolimus containing nanoparticles. J Control Release. 2005;104(2):337–46.
Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Lehr CM, et al. Design of rolipram-loaded nanoparticles: comparison of two preparation methods. J Control Release. 2001;71(3):297–306.
Lamprecht A, Yamamoto H, Ubrich N, Takeuchi H, Maincent P, Kawashima Y. FK506 microparticles mitigate experimental colitis with minor renal calcineurin suppression. Pharm Res. 2005;22(2):193–9.
Ali H, Weigmann B, Neurath MF, Collnot EM, Windbergs M, Lehr C-M. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release [Internet]. June 10, 2014 [cited April 7, 2015];183:167–77. Retrieved from: http://www.sciencedirect.com/science/article/pii/S0168365914001849.
Weiss B, Schaefer UF, Zapp J, Lamprecht A, Stallmach A, Lehr C-M. Nanoparticles made of fluorescence-labelled Poly(L-lactide-co-glycolide): preparation, stability, and biocompatibility. J Nanosci Nanotechnol. 2006;6(9–10):3048–56.
Leroux JC, Allemann E, Doelker E, Gurny R. New approach for the preparation of nanoparticles by an emulsification-diffusion method. Eur J Pharm Biopharm. 1995;41:14–8.
Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6.
Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18(6):788–93.
Jonsson G, Astrom A, Andersson P. Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metab Dispos. 1995;23:137–42.
Sahana DK, Mittal G, Bhardwaj V, Kumar M. PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J Pharm Sci. 2008;57:1530–42.
Tajber L, Corrigan DO, Corrigan OI, Healy AM. Spray drying of budesonide, formoterol fumarate and their composites—I. Physicochemical characterisation. Int J Pharm [Internet]. February 9, 2009 [cited May 5, 2015];367(1–2):79–85. Retrieved from: http://www.sciencedirect.com/science/article/pii/S0378517308006613.
Velaga SP, Berger R, Carlfors J. Supercritical fluids crystallization of budesonide and flunisolide. Pharm Res. 2002;19(10):1564–71.
ACKNOWLEDGMENTS AND DISCLOSURES
Dr. Salman Khan is thanked here for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, H., Weigmann, B., Collnot, EM. et al. Budesonide Loaded PLGA Nanoparticles for Targeting the Inflamed Intestinal Mucosa—Pharmaceutical Characterization and Fluorescence Imaging. Pharm Res 33, 1085–1092 (2016). https://doi.org/10.1007/s11095-015-1852-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1852-6