Abstract
Purpose
To investigate the relationship between viscosity of concentrated MAb solutions and particle size parameters obtained from small-angle X-ray scattering (SAXS).
Methods
The viscosity of three MAb solutions (MAb1, MAb2, and MAb3; 40–200 mg/mL) was measured by electromagnetically spinning viscometer. The protein interactions of MAb solutions (at 60 mg/mL) was evaluated by SAXS. The phase behavior of 60 mg/mL MAb solutions in a low-salt buffer was observed after 1 week storage at 25°C.
Results
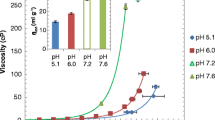
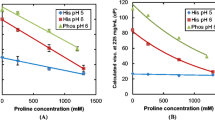
The MAb1 solutions exhibited the highest viscosity among the three MAbs in the buffer containing 50 mM NaCl. Viscosity of MAb1 solutions decreased with increasing temperature, increasing salt concentration, and addition of amino acids. Viscosity of MAb1 solutions was lowest in the buffer containing histidine, arginine, and aspartic acid. Particle size parameters obtained from SAXS measurements correlated very well with the viscosity of MAb solutions at 200 mg/mL. MAb1 exhibited liquid–liquid phase separation at a low salt concentration.
Conclusions
Simultaneous addition of basic and acidic amino acids effectively suppressed intermolecular attractive interactions and decreased viscosity of MAb1 solutions. SAXS can be performed using a small volume of samples; therefore, the particle size parameters obtained from SAXS at intermediate protein concentration could be used to screen for low viscosity antibodies in the early development stage.





Similar content being viewed by others
Change history
24 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11095-023-03479-z
Abbreviations
- D max app :
-
Apparent maximum dimension
- EMS:
-
Electromagnetically spinning
- IFT:
-
Indirect fourier transformation
- p(r):
-
Pair–distance distribution function
- q.s.:
-
quantity sufficient
- R g app :
-
Apparent radius of gyration
- SAXS:
-
Small-angle X-ray scattering
References
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93:1390–402.
Liu J, Nguyen MDH, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94:1928–40.
Kanai S, Liu J, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab–Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97:4219–27.
Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99:1152–68.
Yadav S, Sreedhara A, Kanai S, Liu J, Lien S, Lowman H, et al. Establishing a link between amino acid sequences and self-associating and viscoelastic behavior of two closely related monoclonal antibodies. Pharm Res. 2011;28:1750–64.
Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G. Potential inaccurate quantitation and sizing of protein aggregates by size exclusion chromatography: essential need to use orthogonal methods to assure the quality of therapeutic protein products. J Pharm Sci. 2010;99:2200–8.
Fukunaga K, Onuki M, Ohtsuka Y, Hirano T, Sakai K, Ohgoe Y, et al. Blood viscometer applying electromagnetically spinning method. J Artif Organs. 2013;16:359–67.
Glatter O. Evaluation of small-angle scattering data from lamellar and cylindrical particles by the indirect transformation method. J Appl Cryst. 1980;13:577–84.
Mosbæk CR, Konarev PV, Svergun DI, Rischel C, Vestergaard B. High concentration formulation studies of an IgG2 antibody using small angle X-ray scattering. Pharm Res. 2012;29:2225–35.
Lilyestrom WG, Shire SJ, Scherer TM. Influence of the cosolute environment on IgG solution structure analyzed by small-angle X-ray scattering. J Phys Chem B. 2012;116:9611–8.
Tian X, Langkilde AE, Thorolfsson M, Rasmussen HB, Vestergaard B. Small-angle X-ray scattering screening complements conventional biophysical analysis: comparative structural and biophysical analysis of monoclonal antibodies IgG1, IgG2, and IgG4. J Pharm Sci. 2014;103:1701–10.
Singh SN, Yadav S, Shire SJ, Kalonia DS. Dipole-dipole interaction in antibody solutions: correlation with viscosity behavior at high concentration. Pharm Res. 2014. doi:10.1007/s11095-014-1352-0.
Nishi H, Miyajima M, Nakagami H, Noda M, Uchiyama S, Fukui K. Phase separation of an IgG1 antibody solution under a low ionic strength condition. Pharm Res. 2010;27:1348–60.
Salinas BA, Sathish HA, Bishop SM, Harn N, Carpenter JF, Randolph TW. Understanding and modulating opalescence and viscosity in a monoclonal antibody formulation. J Pharm Sci. 2010;99:82–93.
Arakawa T, Ejima D, Tsumoto K, Obeyama N, Tanaka Y, Kita Y, et al. Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. Biophys Chem. 2007;127:1–8.
Golovanov AP, Hautbergue GM, Wilson SA, Lian LY. A simple method for improving protein solubility and long- term stability. J Am Chem Soc. 2004;126:8933–9.
Shukla D, Trout BL. Understanding the synergistic effect of arginine and glutamic acid mixtures on protein solubility. J Phys Chem B. 2011;115:11831–9.
Fukuda M, Kameoka D, Torizawa T, Saitoh S, Yasutake M, Imaeda Y, et al. Thermodynamic and fluorescence analyses to determine mechanisms of IgG1 stabilization and destabilization by arginine. Pharm Res. 2014;31:992–1001.
Saito S, Hasegawa J, Kobayashi N, Kishi N, Uchiyama S, Fukui K. Behavior of monoclonal antibodies: relation between the second virial coefficient (B2) at low concentrations and aggregation propensity and viscosity at high concentrations. Pharm Res. 2012;29:397–410.
Yadav S, Shire SJ, Kalonia DS. Viscosity behavior of high-concentration monoclonal antibody solutions: correlation with interaction parameter and electroviscous effects. J Pharm Sci. 2012;101:998–1011.
Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, et al. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78.
Cardinaux F, Zaccarelli E, Stradner A, Bucciarelli S, Farago B, Egelhaaf SU, et al. Cluster-driven dynamical arrest in concentrated lysozyme solutions. J Phys Chem B. 2011;115:7227–37.
Yearley EJ, Godfrin PD, Perevozchikova T, Zhang H, Falus P, Porcar L, et al. Observation of small cluster formation in concentrated monoclonal antibody solutions and its implications to solution viscosity. Biophys J. 2014;106:1763–70.
Zhang F, Skoda MW, Jacobs RM, Martin RA, Martin CM, Schreiber F. Protein interactions studied by SAXS: effect of ionic strength and protein concentration for BSA in aqueous solutions. J Phys Chem B. 2007;111:251–9.
Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B. 2013;117:6373–84.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Dr. Kohei Tsumoto (Medical Proteomics Laboratory, Institute of Medical Science, The University of Tokyo) for thoughtful discussions. The authors also thank Akira Hayasaka, Masaru Muraoka, and Yuji Hori in the Discovery Research Department of Chugai Pharmaceutical Co., Ltd. for their support with viscosity measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original article has been corrected. The expression “I(r)” has changed to “I(q)” in an equation in page 3805. The supplementary files have been updated to remove the revision history.
Rights and permissions
About this article
Cite this article
Fukuda, M., Moriyama, C., Yamazaki, T. et al. Quantitative Correlation between Viscosity of Concentrated MAb Solutions and Particle Size Parameters Obtained from Small-Angle X-ray Scattering. Pharm Res 32, 3803–3812 (2015). https://doi.org/10.1007/s11095-015-1739-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1739-6