Abstract
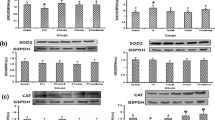
In the present study, regulatory role of vitamin E and curcumin on antioxidant gene (AOG) expression in hypothyroid rat liver is reported. Adult male rats were rendered hypothyroid by administration of 0.05 % 6-propyl-thiouracil in their drinking water, while vitamin E (200 mg/kg body weight) and curcumin (30 mg/kg body weight) were supplemented orally for 30 days. Expression of antioxidant genes (Cu/Zn-superoxide dismutase; SOD1, Mn superoxide dismutase; SOD2, catalase; CAT, glutathione peroxidase; GPx1 and glutathione reductase; GR) was evaluated using RT-PCR and Western blot analyses. The activities of antioxidant enzymes were measured in mitochondrial fraction (MF) and post-mitochondrial fraction (PMF) of rat liver. In addition measurement of glutathione redox status was also carried out in both the fractions. The enhanced transcripts of CAT, GPx1 and GR in hypothyroid rat liver were alleviated by administration of vitamin E and curcumin. Elevated levels of translated product of all AOGs in hypothyroid group were remained unchanged after antioxidant administration. However, enhanced SOD1, GPx1 and decreased GR activities in PMF were normalized by vitamin E and curcumin. Similarly the increased SOD2, GPx1 and decreased CAT activities in MF were also normalized by vitamin E and curcumin supplementation. Administration of vitamin E and curcumin enhanced mitochondrial GSH level; whereas the enhanced GSH level in PMF of hypothyroid rats was alleviated by vitamin E. Thus it can be concluded that besides the antioxidant role of vitamin E and curcumin, they also regulate hepatic antioxidant gene expression in hypothyroid rats.








Similar content being viewed by others
References
Jezek P, Hlavata L (2005) Mitochondria in homeostasis of reactive oxygen species in cell, tissues and organism. Int J Biochem Cell Biol 37:2478–2503
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Halliwell B, Gutteridge JMC (2001) Free radicals in biology and medicine, 3rd edn. Oxford University Press, New York
Venditti P, Di Meo S (2006) Thyroid hormone-induced oxidative stress. Cell Mol Life Sci 63:414–434
Fernandez-Vizarra E, Enriquez JA, Perez-Martos A, Montoya J, Fernandez-Silva P (2008) Moitochondrial gene expression is regulated at multiple levels and differentially in the heart and liver by thyroid hormones. Curr Genet 54:13–22
Pereira B, Rosa LF, Safi DA, Bechara EJ, Curi R (1994) Control of superoxide dismutase, catalase and glutathione peroxidase activities in rat lymphoid organs by thyroid hormones. J Endocrnol 140:73–77
Moro L, Marra E, Capuano F, Greco M (2004) Thyroid hormone treatment of hypothyroid rats restores the regenerative capacity and the mitochondrial membrane permeability properties of the liver after partial hepatectomy. Endocrinology 145:5121–5128
Sanghvi U, Diwakar KK (2008) Universal newborn screening for congenital hypothyroidism. Indian Pediatr 45:331–332
Subudhi U, Das K, Paital B, Bhanja S, Chainy GBN (2009) Supplementation of curcumin and vitamin E enhances oxidative stress, but restores hepatic histoarchitecture in hypothyroid rats. Life Sci 84:372–379
Resch U, Helsel G, Tatzber F, Sinzinger H (2002) Antioxidant status in thyroid dusfunctions. Clin Chem Lab Med 40:1132–1134
Duntas LH (2005) Oxidants, antioxidants in physical exercise and relation to thyroid function. Horm Metab Res 37:572–576
Kiya Y, Miura S, Zhang B, Urata H, Saku K (2006) Effect of levothyroxine on total lipid profiles as assessed by analytical capillary isotachophoresis in a patient with hypothyroidism. Endocrine J 53:865–868
Gerenova J, Gadjeva V (2007) Oxidative stress and antioxidant enzyme activities in patients with Hashimoto’s thyroiditis. Comp Clin Pathol 16:259–264
Mogulkoc R, Baltaci AK, Aydin L, Oztekin E, Sivrikaya A (2005) The effect of thyroxine administration on lipid peroxidation in different tissues of rats with hypothyroidism. Acta Physiol Hung 92:39–46
Mogulkoc R, Baltaci AK, Oztekin E, Ozturk A, Sivrikaya A (2005) Short-term thyroxine administration leads to lipid peroxidation in renal and testicular tissues of rats with hypothyroidism. Acta Biol Hung 56:225–232
Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA (1997) Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned? J Clin Endocrinol Metab 82:1727–1733
Zingg JM (2007) Vitamin E: an overview of major research directions. Mol Aspects Med 28:400–422
Aggarwal BB, Harikumar KB (2009) Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 41:40–59
Subudhi U, Das K, Paital B, Bhanja S, Chainy GBN (2008) Alleviation of enhanced oxidative stress and oxygen consumption of l-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem Biol Interact 173:105–114
Subudhi U, Chainy GBN (2010) Expression of hepatic antioxidant genes in l-thyroxine-induced hyperthyroid rats: regulation by vitamin E and cucumin. Chem Biol Interact 183:304–316
Jena S, Chainy GBN, Dandapat J (2012) Expression of antioxidant genes in renal cortex of PTU-induced hypothyroid rats: effect of vitamin E and curcumin. Mol Biol Rep 39:1193–1203
Das K, Chainy GBN (2001) Modulation of rat liver antioxidant defence system by thyroid hormone. Biochim Biophys Acta 1537:1–13
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation of superoxide radicals. Indian J Biochem Biophys 37:201–204
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutase (SOD) in rat liver: CuZn-SOD in mitochondria. J Biol Chem 276:38388–38393
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Carmagnol F, Sinet PM, Jerome H (1983) Selenium-dependent and non-selenium-dependent glutathione peroxidases in human tissue extracts. Biochim Biophys Acta 759:49–57
Massey V, Williams CH (1965) On the reaction mechanism of yeast glutathione reductase. J Biol Chem 240:4470–4481
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Olsvik PA, Kristensen T, Waagbo R, Rosseland BO, Tollefsen KE, Baeverfjord G, Berntssen MHG (2005) mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp Biochem Physiol Part C 141:314–323
Malik R, Hodgson H (2002) The relationship between the thyroid gland and the liver. Q J Med 95:559–569
Nohl H, Gille L, Staniek K (2004) The mystery of reactive oxygen species derived from cell respiration. Acta Biochim Pol 51:223–229
Sarkar M, Varshney R, Chopra M, Sekhri T, Adhikari JS, Dwarakanath BS (2006) Flow cytometric analysis of reactive oxygen species in peripheral blood mononuclear cells of patients with thyroid dysfunction. Cytometry Part B 70:20–23
Yamakura F, Kawasaki H (2010) Post-translational modifications of superoxide dismutase. Biochim Biophys Acta 1804:318–325
Chung DJ, Wright AE, Clerch LB (1998) The 3′ untranslated region of manganese superoxide dismutase RNA contains a translational enhancer element. Biochemistry 37:16298–16306
Li S, Yan T, Yang JQ, Oberley TD, Oberley LW (2000) The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Caner Res 60:3927–3939
Brigelius-Flohe R (2006) Glutathione peroxidases and redox-regulated transcription factors. Biol Chem 387:1329–1335
Allen RG, Tresini M (2000) Oxidative stress and gene regulation. Free Radic Biol Med 28:463–499
Tapia G, Fernandez V, Varela P, Conejo P, Guerrero J, Videla LA (2003) Thyroid hormone-induced oxidative stress triggers nuclear factor-kappaB activation and cytokine gene expression in rat liver. Free Radic Biol Med 35:257–265
Chattopadhyay S, Sahoo DK, Subudhi U, Chainy GBN (2007) Differential expression profiles of antioxidant enzymes and glutathione redox status in hyperthyroid rats: a temporal analysis. Comp Biochem Physiol Part C 146:383–391
Brent AG (2000) Tissue specific actions of thyroid hormones: insight from animal models. Rev Endocr Metab Dis 1:27–33
Sahoo DK, Roy A, Bhanja S, Chainy GBN (2008) Hypothyroidism impairs antioxidant defence system and testicular physiology during development and maturation. Gen Comp Endocrinol 156:63–70
Cao C, Leng Y, Kufe D (2003) Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J Biol Chem 278:29667–29675
Cao C, Leng Y, Liu X, Yi Y, Li P, Kufe D (2003) Catalase is regulated by ubiquitination and proteosomal degradation. Role of the c-Abl and Arg tyrosine kinases. Biochemistry 42:10348–10353
Itoh M, Oh-Ishi S, Hatao H, Leeuwenburgh C, Selman C, Ohno H, Kizaki T, Nakamura H, Matsuoka T (2004) Effects of dietary calcium restriction and acute exercise on the antioxidant enzyme system and oxidative stress in rat diaphragm. Am J Physiol Regul Integr Comp Physiol 287:R33–R38
Cao C, Leng Y, Huang W, Liu X, Kufe D (2003) Glutathione peroxidase 1 activity is regulated by c-Abl and Arg tyrosine kinases. J Biol Chem 278:39609–39614
Korkina L, Deeva I, Cesareol E, De Luca C (2009) The chemical defensive system in the pathobiology of idiopathic environment-associated diseases. Curr Drug Metab 10:914–931
Zoltan U, Sonntag WE, Csiszar A (2010) Mitochondria and aging in the vascular system. J Mol Med 88:1021–1027
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921
Messarah M, Boulakoud MS, Boumendjel A, Abdennour C, Feki AE (2007) The impact of thyroid activity variations on some oxidizing-stress parameters in rats. CR Biol 330:107–112
Lu SC, Kuhlenkamp J, Garcia-Ruiz C, Kaplowitz N (1991) Hormone-mediated down-regulation of hepatic glutathione synthesis in the rat. J Clin Invest 88:260–269
Zheng S, Yumei F, Chen A (2007) De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med 43:444–453
Fridovich I (1978) The biology of oxygen radicals. Science 201:875–880
Jones DP (2002) Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348:93–112
Sarandol E, Tas S, Dirican M, Serdar Z (2005) Oxidative stress and serum paraoxonase activity in experimental hypothyroidism: effect of vitamin E supplementation. Cell Biochem Funct 23:1–8
Erdamar H, Demirci H, Yaman H, Erbil MK, Yakar T, Sancak B, Elbeg S, Biberoglu G, Yetkin I (2008) The effect of hypothyroidism, hyperthyroidism and their treatment on parameters of oxidative stress and antioxidant status. Clin Chem Lab Med 46:1004–1010
Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI (2008) Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta 1780:673–679
Acknowledgments
This work was supported by the Indian Council of Medical Research, Department of Biotechnology and Department of Science and Technology, Government of India, New Delhi.
Conflict of interest
No conflict to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subudhi, U., Chainy, G.B.N. Curcumin and vitamin E modulate hepatic antioxidant gene expression in PTU-induced hypothyroid rats. Mol Biol Rep 39, 9849–9861 (2012). https://doi.org/10.1007/s11033-012-1851-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1851-1