Abstract
Anthocyanins, natural pigments with high antioxidant activities, are widely distributed in the plant kingdom and play important roles in various physiological processes. Much effort has been committed to enhancing the anthocyanin content of these health-promoting pigments in vegetables and grains, for their eye-catching colors and special nutrients. Previously, we reported that the SmMYB1 gene encoding a R2R3 MYB transcription factor participated in the regulation of anthocyanin biosynthesis in the peel of eggplant. In this work, we introduced SmMYB1 into a non-anthocyanin-accumulating eggplant cultivar (Solanum aethiopicum group Gilo) via Agrobacterium-mediated transformation. Genetically engineered plants exhibited high concentrations of anthocyanin in leaves, petals, stamens, and fruit peels under normal growth conditions, especially in fruit flesh. Furthermore, highly methylated anthocyanins, malvidin 3-(p-coumaroyl)rhamnoside(glucoside)-5-glucoside and malvidin 3-(feruloyl)rhamnoside(glucoside)-5-glucoside, were separated from the purple fruit flesh and identified by HPLC–ESI–MS/MS. qRT-PCR analysis revealed that most anthocyanin structural genes were dramatically up-regulated in the tissues of transgenic lines compared with non-transformed plants. In addition, the transgenic seedlings had greater tolerance to freezing stress and better recovery under rewarming conditions. These results provide a good foundation for the breeding of new eggplant cultivars with more healthy agronomic traits in future studies.




Similar content being viewed by others
Abbreviations
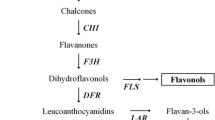
- PAL:
-
Phenylalanine ammonia lyase
- C4H:
-
Cinnamate 4-hydroxylase
- 4CL:
-
4-Coumarate-CoA ligase
- CHS:
-
Chalcone synthase
- CHI:
-
Chalcone isomerase
- F3H:
-
Flavone 3-hydroxylase
- F3′H:
-
Flavonoid 3′-hydroxylase
- DFR:
-
Dihydroflavonol reductase
- ANS:
-
Anthocyanidin synthase
- UFGT:
-
Flavonoid-3-O-glucosyltransferase
- GST:
-
Glutathione S-transferase
- OMT:
-
O-Methyltransferase
- DHK:
-
Dihydrokaempferol
- DHQ:
-
Dihydroquercetin
- DHM:
-
Dihydromyricetin
- HPLC:
-
High-performance liquid chromatography
- ESI–MS/MS:
-
Electrospray ionization tandem mass spectrometry
- qRT-PCR:
-
Quantitative real-time PCR
References
Ahmed NU, Park J-I, Jung H-J, Hur Y, Nou I-S (2014) Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct Integr Genomic 15:383–394
Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM (2011) Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J Cell Mol Biol 65:771–784
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48:958–970
Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39:366–380
Baudry A, Caboche M, Lepiniec L (2006) TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J Cell Mol Biol 46:768–779
Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132:291–298
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2393
Bovy A (2002) High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell Online 14:2509–2526
Bradshaw HD, Schemske DW (2003) Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426:176–178
Broun P (2005) Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr Opin Plant Biol 8:272–279
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EG, Hall RD, Bovy AG, Luo J, Martin C (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26:1301–1308
Cao G, Sofic E, Prior RL (1996) Antioxidant capacity of tea and common vegetables. J Agric Food Chem 44:3426–3431
Chiu LW, Zhou X, Burke S, Wu X, Prior RL, Li L (2010) The purple cauliflower arises from activation of a MYB transcription factor. Plant Physiol 154:1470–1480
de Pascual-Teresa S, Sanchez-Ballesta MT (2007) Anthocyanins: from plant to health. Phytochem Rev 7:281–299
Espley RV, Bovy A, Bava C, Jaeger SR, Tomes S, Norling C, Crawford J, Rowan D, McGhie TK, Brendolise C, Putterill J, Schouten HJ, Hellens RP, Allan AC (2013) Analysis of genetically modified red-fleshed apples reveals effects on growth and consumer attributes. Plant Biotechnol J 11:408–419
Filippone E, Lurquin P (1989) Stable transformation of eggplant (Solanum melongena L.) by cocultivation of tissues with Agrobacterium tumefaciens carrying a binary plasmid vector. Plant Cell Rep 8:370–373
Goff SA, Cone KC, Chandler VL (1992) Functional analysis of the transcriptional activator encoded by the maize B gene: evidence for a direct functional interaction between two classes of regulatory proteins. Gene Dev 6:864–875
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J Cell Mol Biol 53:814–827
Gonzali S, Mazzucato A, Perata P (2009) Purple as a tomato: towards high anthocyanin tomatoes. Trends Plant Sci 14:237–241
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57:761–780
Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK (2006) Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 142:98–112
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Holton TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7:1071–1083
Honda C, Kotoda N, Wada M, Kondo S, Kobayashi S, Soejima J, Zhang Z, Tsuda T, Moriguchi T (2002) Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol Biochem 40:955–962
Jaakola L (2013) New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci 18:477–483
Jaakola L, Poole M, Jones MO, Kamarainen-Karppinen T, Koskimaki JJ, Hohtola A, Haggman H, Fraser PD, Manning K, King GJ, Thomson H, Seymour GB (2010) A SQUAMOSA MADS box gene involved in the regulation of anthocyanin accumulation in bilberry fruits. Plant Physiol 153:1619–1629
Jung CS, Griffiths HM, De Jong DM, Cheng S, Bodis M, Kim TS, De Jong WS (2009) The potato developer (D) locus encodes an R2R3 MYB transcription factor that regulates expression of multiple anthocyanin structural genes in tuber skin. Theor Appl Genet 120:45–57
Klaper R, Frankel S, Berenbaum MR (1996) Anthocyanin content and UVB sensitivity in Brassica rap. Photochem Photobiol 63:811–813
Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10:236–242
Lalusin A, Nishita K, Kim S-H, Ohta M, Fujimura T (2006) A new MADS-box gene (IbMADS10) from sweet potato (Ipomoea batatas (L.) Lam) is involved in the accumulation of anthocyanin. Mol Genet Genomics 275:44–54
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430
Li HM, Rotter D, Hartman TG, Pak FE, Havkin-Frenkel D, Belanger FC (2006) Evolution of novel O-methyltransferases from the Vanilla planifolia caffeic acid O-methyltransferase. Plant Mol Biol 61:537–552
Mano H, Ogasawara F, Sato K, Higo H, Minobe Y (2007) Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol 143:1252–1268
Mathews H (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell Online 15:1689–1703
Mendez M, Jones DG, Manetas Y (1999) Enhanced UV-B radiation under field conditions increases anthocyanin and reduces the risk of photoinhibition but does not affect growth in the carnivorous plant Pinguicula vulgaris. New Phytol 144:275–282
Meng X, Yin B, Feng H-L, Zhang S, Liang X-Q, Meng Q-W (2014) Overexpression of R2R3-MYB gene leads to accumulation of anthocyanin and enhanced resistance to chilling and oxidative stress. Biol Plantarum 58:121–130
Mooney M, Desnos T, Harrison K, Jones J, Carpenter R, Coen E (1995) Altered regulation of tomato and tobacco pigmentation genes caused by the delila gene of Antirrhinum. Plant J 7:333–339
Morita Y, Saitoh M, Hoshino A, Nitasaka E, Iida S (2006) Isolation of cDNAs for R2R3-MYB, bHLH and WDR transcriptional regulators and identification of c and ca mutations conferring white flowers in the Japanese morning glory. Plant Cell Physiol 47:457–470
Park KI, Ishikawa N, Morita Y, Choi JD, Hoshino A, Iida S (2007) A bHLH regulatory gene in the common morning glory, Ipomoea purpurea, controls anthocyanin biosynthesis in flowers, proanthocyanidin and phytomelanin pigmentation in seeds, and seed trichome formation. Plant J Cell Mol Biol 49:641–654
Park NI, Xu H, Li X, Jang IH, Park S, Ahn GH, Lim YP, Kim SJ, Park SU (2011) Anthocyanin accumulation and expression of anthocyanin biosynthetic genes in radish (Raphanus sativus). J Agric Food Chem 59:6034–6039
Rapisarda P, Fanella F, Maccarone E (2000) Reliability of analytical methods for determining anthocyanins in blood orange juices. J Agric Food Chem 48:2249–2252
Schwinn K, Venail J, Shang Y, Mackay S, Alm V, Butelli E, Oyama R, Bailey P, Davies K, Martin C (2006) A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. Plant Cell 18:831–851
Shi MZ, Xie DY (2014) Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent Pat Biotechnol 8:47–60
Shin Y-M, Park H-J, Yim S-D, Baek N-I, Lee C-H, An G, Woo Y-M (2006) Transgenic rice lines expressing maize C1 and R-S regulatory genes produce various flavonoids in the endosperm. Plant Biotechnol J 4:303–315
Spelt C, Quattrocchio F, Mol JN, Koes R (2000) Anthocyanin1 of petunia encodes a basic helix–loop–helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 12:1619–1632
Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142:1216–1232
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749
Toppino L, Valè G, Rotino GL (2008) Inheritance of Fusarium wilt resistance introgressed from Solanum aethiopicum Gilo and Aculeatum groups into cultivated eggplant (S. melongena) and development of associated PCR-based markers. Mol Breed 22:237–250
Tsuda T, Horio F, Osawa T (2000) The role of anthocyanins as an antioxidant under oxidative stress in rats. BioFactors 13:133–139
Van der Krol AR, Mur LA, Beld M, Mol J, Stuitje AR (1990) Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell Online 2:291–299
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Wu X, Prior RL (2005) Identification and characterization of anthocyanins by high-performance liquid chromatography–electrospray ionization-tandem mass spectrometry in common foods in the United States: vegetables, nuts, and grains. J Agric Food Chem 53:3101–3113
Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ (2012) The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ 35:1884–1897
Yakimchuk R, Hoddinott J (1994) The influence of ultraviolet-B light and carbon dioxide enrichment on the growth and physiology of seedlings of three conifer species. Can J For Res 24:1–8
Yuan Y, Chiu LW, Li L (2009) Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta 230:1141–1153
Zhang Y, Butelli E, Martin C (2014a) Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol 19:81–90
Zhang Y, Chen G, Dong T, Pan Y, Zhao Z, Tian S, Hu Z (2014b) Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in Purple Bok Choy (Brassica rapa var. Chinensis). J Agric Food Chem 62:12366–12376
Zhang Y, Hu Z, Chu G, Huang C, Tian S, Zhao Z, Chen G (2014c) Anthocyanin accumulation and molecular analysis of anthocyanin biosynthesis-associated genes in eggplant (Solanum melongena L.). J Agric Food Chem 62:2906–2912
Zhang Y, Hu Z, Zhu M, Zhu Z, Wang Z, Tian S, Chen G (2015) Anthocyanin accumulation and molecular analysis of correlated genes in Purple Kohlrabi (Brassica oleracea var. gongylodes L.). J Agric Food Chem 63:4160–4169
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 31572129), the Fundamental Research Funds for the Central Universities (No. 106112015CDJZR235504), and Technology System of National Bulk Vegetable Industry–Eggplant Breeding Position (CARS-25-A-06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanjie Zhang and Guihua Chu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Chu, G., Hu, Z. et al. Genetically engineered anthocyanin pathway for high health-promoting pigment production in eggplant. Mol Breeding 36, 54 (2016). https://doi.org/10.1007/s11032-016-0454-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-016-0454-2