Abstract
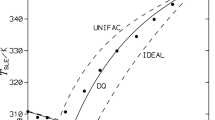
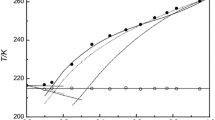
Solid–liquid equilibria of the n-alkanes (n-octadecane, n-eicosane, n-tetracosane, n-pentacosane, n-triacontane) in biphenyl were measured by DSC 7 (Perkin-Elmer). It was found that all systems are simple eutectic. The solubility of the biphenyl in n-alkanes was studied in the temperature range 301–370 K. The experimental results were correlated using modified UNIFAC (Larsen and Gmehling versions) and ideal models. Good representation of solubility diagrams was obtained using partly readjusted UNIFAC parameters of Larsen version. Taking into account the large range of applicability of UNIFAC and the predictions of the activity coefficients for many other components in different classes of mixtures, we can conclude that the new experimental data for the systems mentioned in this work should be included in the database used by UNIFAC in order to evaluate better interaction parameters and to improve predictions.












Similar content being viewed by others
References
Jennings DW, Weispfennig K. Experimental solubility data of various n-alkane waxes: effects of alkane chain length, alkane odd versus even carbon number structures and solvent chemistry on solubility. Fluid Phase Equilib. 2005;227:27–35.
Burger ED, Perkins TK, Striegler JH. Studies of wax deposition in the trans. Alaska pipeline. J Petroleum Technol. 1981;33:1075–86.
Rønningsen HP, Bjørndal B, Hansen AB, Pedersen WB. Wax precipitation from North Sea oils. 1. Crystallization and dissolution temperature, and Newtonian and non-Newtonian flow properties. Energy Fuels. 1991;5:895–908.
Hammami A, Raines MA. Paraffin deposition from crude oils: comparison of laboratory results to field data. SPE J. 1999;4(1):9–18.
Coutinho JAP, Edmonds B, Moorwood T, Szczepanski R, Zhang X. Reliable wax predictions for flow assurance. Energy Fuels. 2006;20:1081–8.
Djordjevic NM. Solubilities of polycyclic aromatic hydrocarbon solids in n-octadecane. Thermochim Acta. 1991;177:109–18.
Aoulmi A, Bouroukba M, Solimando R, Rogalski M. Thermodynamics of mixtures formed by polycyclic aromatic hydrocarbons with long chain alkanes. Fluid Phase Equilib. 1995;110:283–97.
Mahmoud R, Solimando R, Rogalski M. Solid–liquid equilibria of systems containing pyrene and long chain normal-alkanes. Fluid Phase Equilib. 1998;148:139–46.
Mahmoud R, Solimando R, Bouroukba M, Rogalski M. Solid–liquid equilibrium and excess enthalpy measurements in binary dibenzofuran or xanthene + normal long-chain alkane systems. J Chem Eng Data. 2000;45:433–6.
Hafsaoui SL, Mahmoud R. Solid-liquid equilibria of binary systems containing n-tetracosane with naphthalene or dibenzofuran prediction with UNIFAC model. J Therm Anal Calorim. 2007;88:565–70.
Larsen BL, Rasmussen P, Fredenslund A. A modified UNIFAC group-contribution model for prediction of phase equilibria and heats of mixing. Ind Eng Chem Res. 1987;26:2274–86.
Weidlich U, Gmehling J. A modified UNIFAC model. 1. Prediction of VLE, hE, and γ∞. Ind Eng Chem Res. 1987;26:1372–81.
Gmehling J, Li J, Schiller M. A modified UNIFAC model. 2. Present parameter matrix and results for different thermodynamic properties. Ind Eng Chem Res. 1993;32:178–93.
Kniaz K. Influence of size and shape effects on the solubility of hydrocarbons: the role of the combinatorial entropy. Fluid Phase Equilib. 1991;68:35–46.
Khimeche K, Boumrah Y, Benziane M, Dahmani A. Solid–liquid equilibria and purity determination for binary n-alkane + naphthalene systems. Thermochim Acta. 2006;444:166–72.
Benziane M, Khimeche K, Dahmani A, Nezar S, Trache D. Experimental determination and prediction of (solid + liquid) phase equilibria for binary mixtures of heavy alkanes and fatty acids. Mol Phys. 2012;110:1383–9.
Company JC. Mesure et interprétation des équilibres de cristallisation de solutions de paraffines lourdes et d’hydrocarbures aromatiques. Chem Eng Sci. 1973;28:318–23.
Mondieig D, Rajabalee F, Metivaud V, Oonk HAJ, Cuevas-Diarte MA. n-Alkane binary molecular alloys. Chem Mater. 2004;16:786–98.
Cabaleiro D, Gracia-Fernandez C, Lugo L. (Solid + liquid) phase equilibria and heat capacity of (diphenyl ether + biphenyl) mixtures used as thermal energy storage materials. J Chem Thermodyn. 2014;74:43–50.
Praunitz JM, Lichtenthaler RN, Azevedo EG. Molecular thermodynamics of fluid phase equilibria. 2nd ed. Engelwood Cliffs: Prentice-Hall; 1986.
Nelder JA, Mead R. A simplex method for function minimization. Comp. J. 1965;7:308–13.
Domanska U, González JA. Thermodynamics of branched alcohols II. Solid–liquid equilibria for systems containing tert-butanol and long-chain n-alkanes. Experimental results and comparison with DISQUAC predictions. Fluid Phase Equilib. 1998;147:251–70.
Domanska U, Szurgocinska M, González JA. Thermodynamics of binary mixtures containing organic carbonates Part XI. SLE measurements for systems of diethyl carbonate with long n-alkanes: comparison with DISQUAC and modified UNIFAC predictions. Fluid Phase Equilib. 2001;190:15–31.
Hammami A, Mehrotra AK. Thermal behaviour of polymorphic n-alkanes: effect of cooling rate on the major transition temperatures. Fuel. 1995;74:96–101.
Dirand M, Chevallier V, Provost E, Bouroukba M, Petitjean D. Multicomponent paraffin waxes and petroleum solid deposits: structural and thermodynamic state. Fuel. 1998;77:1253–60.
Chevallier V, Bouroukba M, Petitjean D, Barth D, Dupuis P, Dirand M. Temperatures and enthalpies of solid-solid and melting transitions of the odd-numbered n-alkanes C21, C23, C25, C27, and C29. J Chem Eng Data. 2001;46:1114–22.
Roblès L, Mondieig D, Haget Y, Cuevas-Diarte MA. Mise au point sur le comportement énergétique et cristallographique des n-alcanes. II. Série de C22H46 à C27H56. J Chim Phys Phys Chim Biol. 1998;95:92–111.
Briard AJ, Bouroukba M, Petitjean D, Hubert N, Dirand M. Experimental enthalpy increments from the solid phases to the liquid phase of homologous n-alkane series (C18 to C38 and C41, C44, C46, C50, C54, and C60). J Chem Eng Data. 2003;48:497–513.
Gioia-Lobbia G, Vitali G. Crystallization curves for binary mixtures of alkanes, acids, and alcohols. J Chem Eng Data. 1984;29:16–8.
Dirand M, Achour-Boudjema Z. Structural evolution versus temperature of the βo phase of the n-eicosane/n-docosane system : rotator transitions. J Mol Struct. 1996;375:243–8.
Dirand M, Bouroukba M, Briard AJ, Chevallier V, Petitjean D, Corriou JP. Temperatures and enthalpies of solid + solid and solid + liquid transitions of n-alkanes. J Chem Therm. 2002;34:1255–77.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boudouh, I., Hafsaoui, S.L., Mahmoud, R. et al. Measurement and prediction of solid–liquid phase equilibria for systems containing biphenyl in binary solution with long-chain n-alkanes. J Therm Anal Calorim 125, 793–801 (2016). https://doi.org/10.1007/s10973-016-5407-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5407-9