Abstract
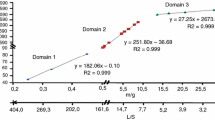
Kinetics of the attack of a Tunisian phosphate ore by phosphoric acid solution was calorimetrically investigated using a differential reaction calorimeter. Determination of the time constants (τ 1 and τ 2) and the transfer function of this device allowed calculation of the thermogenesis curves which were used for the kinetic study at different temperatures. It was found that the attack rate increased with increasing temperature and the kinetic results agree with the shrinking-core model with an ash layer diffusion control. The resulting apparent activation energy equals 25.4 ± 1.8 kJ mol−1, which is in the range determined by the isoconversional model (11.1–26.3 kJ mol−1).









Similar content being viewed by others
References
Hsieh SS. Beneficiation of dolomotic phosphate ores using modified Crago-TVA process. Ind Eng Chem Res. 1987;26:1413–9.
Mostafa SI, Sadda MY, Boulis SN, Hawash SI. Flotation of low grade siliceous calcareous phosphate ore. Physicochem Probl Miner Process. 1998;42:5–16.
Al-Fariss TF, Ozbelge HO, El-Shall HS. On the phosphate rock beneficiation for the production of phosphoric acid in Saudi Arabia. J King Saud Univ Eng Sci. 1992;4:13–32.
Guimarâes RC, Araujo AC, Peres AEC. Reagent in igneous phosphate ores flotation. Miner Eng. 2005;18:199–204.
Chaabouni A, Chtara C, Nzihou A, El-Feki H. Kinetic study of the dissolution of Tunisian natural phosphate or francolite in industrial phosphoric acid. J Adv Chem. 2013;6:908–13.
Dorozohkin SV. Dissolution Kinetics of Single Fluorapatite Crystals in Phosphoric Acid Solution under the Conditions of the Wet-Process, Phosphoric Acid Production. J Prakt Chem. 1996;338:620–6.
Hamdi AS, Remedhan ST. Abd Ali H. Phosphate rock treatment with hydrochloric acid for increasing P2O5. J Eng Technol. 2012;30:67–76.
Ashraf M, Zafar ZI, Ansari TM, Ahmed F. Selective leaching kinetics of calcareous phosphate rock in phosphoric acid. J Appl Sci. 2005;5:1722–7.
Mizane A, Louhi A. Comparative study of the dissolution of phosphate rock of Djebel Onk (Algéria) by the nitric acid and the sulfuric acid. J Eng Appl Sci. 2007;2:1016–9.
Aly HF, Ali MM, Taha MH. Dissolution kinetics of Western Desert phosphate rocks, Abu Tartur with hydrochloric acid. Arab Journal of Nuclear Science and Applications. 2013;46:1–16.
Sluis SV, Meszaros Y, Marchee WGJ, Wesselingh HA, Rosmalen GMV. The digestion of phosphate ore in phosphoric acid. Ind Eng Chem Res. 1987;26:2501–5.
Sevim F, Sarac H, Kocakarim MM, Yartasi A. Dissolution kinetics of phosphate ore in H2SO4 solutions. Ind Eng Chem Res. 2003;42:2052–7.
Olanipekun EO. Kinetics of dissolution of phosphorite in acid mixtures. Bull Chem Soc Ethiop. 1999;13:63–70.
Brahim K, Antar K, Khattech I, Jemal M. Effect of temperature on the attack of fluorapatite by a phosphoric acid solution. Sci Res Essays. 2008;3:035–9.
Bayramoglu M, Demircilolu N, Tekin T. Dissolution kinetics of Mazidagi phosphate rock in HNO3 solution. Int J Miner Process. 1995;43:249–54.
Serdyuk VV, Panov VP, Tereshchenko LY, Chekreneva GM. Mechanism of the dissolution of apatite by phosphoric acid in the presence of electrolytes. Zh Prikl Khim. 1982;55:2190.
Huffman EO, Cate WE, Deming ME, Elmore KL. Rates of solution of calcium phosphates in phosphoric acid solutions. J Agric Food Chem. 1957;5:266–75.
Shakourzadeh K, Bloise R, Baratin F. Crystallization of calcium sulfate hemihydrate in reagent-grade phosphoric acid. Ind Miner Tech. 1984;9:72.
Ivanov EV, Zinyuk RY, Pozin ME. Kinetic characteristics of the process of dissolving in phosphoric acid. Zh Prikl Khim. 1977;50:1193.
Brahim K, Antar K, Khattech I, Jemal M. Etude thermodynamique et cinétique de l’attaque de la fluorapatite par l’acide phosphorique. Ann Chim Sci Mat. 2006;31:611–21.
Brahim K, Khattech I, Dubès JP, Jemal M. Etude cinétique et thermodynamique de la dissolution de la fluorapatite dans l’acide phosphorique. Thermochim Acta. 2005;436:43–50.
Antar K, Brahim K, Jemal M. Etude cinétique et thermodynamique de l’attaque d’une fluorapatite par des mélanges d’acides sulfurique et phosphorique à 25 °C. Thermochim Acta. 2006;449:35–41.
Antar K, Jemal M. Kinetics and thermodynamics of the attack of fluorapatite by a mixture of sulfuric and phosphoric acids at 55 °C. Thermochim Acta. 2007;452:71–5.
Antar K, Jemal M. Kinetics and thermodynamics of the attack of a phosphate ore by acid solutions at different temperatures. Thermochim Acta. 2008;474:32–5.
Zendah H, Khattech I, Jemal M. Synthesis, characterization, and thermochemistry of acid attack of “B” type carbonate fluorapatites. J Therm Anal Calorim. 2012;109:855–61.
El Asri S, Laghzizil A, Alaoui A, Saoiabi A, M’Hamdi R, El Abbassi K, Hakam A. Structure and thermal behaviors of Moroccan phosphate rock (Benguerir). J Therm Anal Calorim. 2009;95:15–9.
Fertani-Gmati M. Mecanisme de dissolution de la silice dans des solutions de NaOH Essais de piegeage par la «voie silicate» de Fe, Al et Mg en milieu phosphorique. Thesis, Tunis El Manar University, 2014.
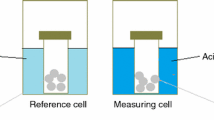
Nogent H, Le Tacon X. The differential reaction calorimeter: a simple apparatus to determine reaction heat, heat transfer value and heat capacity. J Loss Prev. 2002;15:445–8.
Nogent H, Le Tacon X. The differential reaction calorimeter: examples of use. J Loss Prev. 2003;16:133–9.
Soussi-Baatout A, Hichri M, Bechrifa A, Khattech I. Test and calibration processes for the Differential Reaction Calorimeter (DRC): application: Dissolution of Calcium Fluorapatite in the hydrochloric acid. Thermochim Acta. 2014;580:85–92.
Dubès JP. Déconvolution de la réponse instrumentale par filtrage inverse en calorimétrie à Conduction. Thèse de doctorat d’état. Université de Provence, France, 1985.
Ivernel A et al. Représentations analogiques et homologiques dans les techniques de la chaleur. Dunod éd, Paris, 1965.
Calvet E, Camia F. Lobtention des courbes de thermogenese a partir des courbes enregistrees au microcalorimetre de E, CALVET. J Chim Phys PCB. 1958;55:818.
Laville GM. Calorimétrie: théorie générale du microcalorimètre Calvet. C R Acad Sci. 1955;240:1060–195.
Dubès JP, Barrès M, Tachoire H. Calorimétrie: correcteur automatique d’inertie pour calorimètres à conduction et analyseurs calorimétriques différentiels. C R Acad Sci Paris. 1976;283:163–6.
Thouvenin Y, Hinnen C, Rousseau A. In actes du colloque international de microcalorimétrie et de thermogenèse, Marseille, (1965) (CNRS éd., Paris 1967).
Fertani-Gmati M, Brahim K, Khattech I, Jemal M. Thermochemistry and kinetics of silica dissolution in NaOH solutions: effect of the alkali concentration. Thermochim Acta. 2014;594:58–67.
Levenspiel O. Chemical reaction engineering. 3rd ed. New York: Wiley; 1999. p. 566–86.
Sohn H, Wadsworth ME. Rate processes of extractive metallurgy. New York: Plenum press; 1979. p. 141.
Habashi F. Principles of extractive metallurgy. New York: Gordon and Breach; 1979. p. 11.
Tekin G, Onganer Y, Alkan M. Dissolution of ulexite in ammonium chloride solutions. Can Mctall Q. 1998;37:91–7.
Gharabaghi M, Irannajad M, Noaparast M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy. 2010;103:96–107.
Zafar ZI. Determination of semi empirical kinetic model for dissolution of bauxite ore with sulfuric acid: parametric cumulative effect on the Arrhenius parameters. J Chem Eng. 2008;141:233–41.
Souza AD, Pina PS, Leâo VA, Silva CA, Siqueira PF. The leaching kinetics of a zinc sulfide concentrate in acid ferric sulfate. Hydrometallurgy. 2007;89:72–81.
Abdel-Aal EA, Rashed MM. Kinetic study on the leaching of spent nickel oxide catalyst with sulfuric acid. Hydrometallurgy. 2004;74:189–94.
Calmanovici CE, Gilot B, Laguérie C. Mechanism and kinetics for the dissolution of apatitic materials in acid solutions. Braz J Chem Eng. 1997;14:95–102.
Sbirrazzuoli N, Brunel D, Elegant L. Different kinetic equations analysis. J Therm Anal Calorim. 1992;38:1509–24.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402.
Fertani-Gmati M, Jemal M. Thermochemical and kinetic investigations of amorphous silica dissolution in NaOH solutions. Therm Anal Calorim. 2015. doi:10.1007/s10973-015-4980-7.
Vyazovkin S. A unified approach to kinetic processing of nonisothermal data. lnt J Chem Kinet. 1996;28:95–101.
Sbirrazzuoli N, Vyazovkin S. Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim Acta. 2002;388:289–98.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soussi-Baatout, A., Ibrahim, K., Khattech, I. et al. Attack of Tunisian phosphate ore by phosphoric acid. J Therm Anal Calorim 124, 1671–1678 (2016). https://doi.org/10.1007/s10973-016-5263-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5263-7