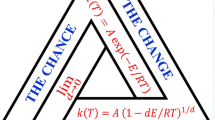
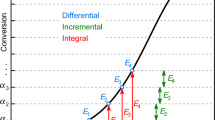
Abstract
A generalized logistic function has been proposed as a kinetic analysis method that is superior to traditional methods. In fact, the parameter τ in the generalized logistic function has an effect on the reaction profile similar to the parameter n in the extended Prout–Tompkins model for autocatalytic reactions. Furthermore, the comparisons made in some papers to traditional methods were made by using the discredited method of determining kinetic parameters from a single heating rate, so they are misleading compared to proper kinetic analysis methods that simultaneously analyze multiple thermal histories. In addition, the current implementation of the generalized logistic function of fitting each experiment individually is prone to introduce errors in the kinetic parameters. Guidance is given on how the generalized logistic function might be used for proper chemical kinetic analysis.







Similar content being viewed by others
References
Verhulst PF. Notice sur la loi que la population suit dans son accroissement. Corresp Math Phys. 1838;10:113–21.
Prout EG, Tompkins FC. The thermal decomposition of potassium permanganate. Trans Faraday Soc. 1944;40:488–98.
Brown ME. The Prout–Tompkins rate equation in solid-state kinetics. Thermochim Acta. 1997;300:93–106.
Lewis GN. Autocatalytic decomposition of silver oxide. Proc Am Acad Arts Sci. 1905;40:719–33.
Austin JB, Rickett RL. Kinetics of the decomposition of austenite at constant temperature. Trans AIME. 1938;135:396–415; AIME Technical publication 964, 20 pp.
Akulov NS. Basics of chemical dynamics. Moscow: Moscow State University; 1940 (in Russian).
Young DA. Thermal decomposition of solids. Oxford: Pergamon; 1966, p. 35.
Burnham AK. Application of the Šesták–Berggren equation to organic and inorganic materials of practical interest. J Therm Anal Calor. 2000;60:895–908.
Burnham AK, Braun RL, Coburn TT, Sandvik EI, Curry DJ, Schmidt BJ, Noble RA. An appropriate kinetic model for well-preserved algal kerogens. Energy Fuels. 1996;10:49–59.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Ng WL. Thermal decomposition in the solid state. Aust J Chem. 1975;28:1169–78.
Prout EG, Tompkins FC. The thermal decomposition of silver permanganate. Trans Far Soc. 1946;42:468–72.
Bawn CEH. The decomposition of organic solids. In: Garner WE, editor. Chemistry of the solid state, Chapter 10. London: Butterworths; 1955. p. 254–67.
Burnham AK, Weese RK, Wemhoff AP, Maienschein JL. A historical and current perspective on predicting thermal cookoff behavior. J Therm Anal Calor. 2007;89:407–15.
Burnham AK, Weese RK. Kinetics of thermal degradation of explosive binders Viton A, Estane, and Kel-F. Thermochim Acta. 2005;426:85–92.
Pérez-Maqueda LA, Criado JM, Sánchez-Jiménez PE. Combined kinetic analysis of solid-state reactions: a powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J Phys Chem A. 2006;110:12456–62.
Burnham AK, Weese RK, Weeks BL. A distributed activation energy model of thermodynamically inhibited nucleation and growth reactions and its application to the beta-delta phase transition of HMX. J Phys Chem B. 2004;108:19432–41.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, et al. Computational aspects of kinetic analysis. Part A: the ICTAC Kinetics Project—data, methods and results. Thermochim Acta. 2000;355:125–43.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Naya S, Cao R, Lopez de Ullibarri I, Artiaga R, Barbadillo F, Garcia A. Logistic mixture versus Arrhenius for kinetic study of material degradation by dynamic thermogravimetric analysis. J Chemom. 2006;20:158–63.
Cao R, Naya S, Artiaga R, Garcia A, Varela A. Logistic approach to polymer degradation in dynamic TGA. Poly Degrad Stab. 2004;85:667–74.
Barbadillo F, Fuentes A, Naya S, Cao R, Mier JL, Artiaga R. Evaluating the logistic mixture model on real and simulated TG curve. J Therm Anal Calorim. 2007;87:223–7.
Rios-Fachal M, Garcia-Fernandez C, Lopez-Beceiro J, Gomez-Barreiro S, Tarrio-Saavedra J, Ponton A, Ariaga R. Effect of nanotubes on the thermal stability of polystyrene. J Therm Anal Calorim. 2013;113:481–7.
Tarrio-Saavedra J, Lopez-Beceiro J, Naya S, Francisco-Fernandez M, Artiaga R. Simulation study for generalized logistic function in thermal data modeling. J Therm Anal Calorim. 2014;118:1253–68.
Burnham AK, Braun RL. Global kinetic analysis of complex materials. Energy Fuels. 1999;13:1–22.
Craido JM, Morales J. Defects of thermogravimetric analysis for discerning between first order reactions and those taking place through the Avrami–Erofeev’s mechanism. Thermochim Acta. 1976;16:382–7.
Arrhenius S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z Phys Chem. 1989;4:226.
Laidler KJ, King MC. The development of transition-state theory. J Phys Chem. 1983;87:2657–64.
Jellinek HHG. Degradation and stabilization of polymers. Amsterdam: Elsevier; 1983.
Flynn JH, Florin RE. Degradation and pyrolysis mechanisms. In: Liebman SA, Levy EJ, editors. Pyrolysis and GC in polymer analysis. New York: Marcel Dekker; 1985. p. 149–208.
Budrugeac P. Theory and practice in the thermoanalytical kinetics of complex processes: application for the isothermal and nonisothermal thermal degradation of HDPE. Thermochim Acta. 2010;500:30–7.
Gotor FJ, Criado JM, Malak J, Koga N. Kinetic analysis of solid-state reactions: the Universality of Master Plots for analyzing isothermal and nonisothermal experiments. J Phys Chem A. 2000;104:10777–82.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C. 1964;(6):183–95.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzouli N, Sunol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Budrugeac P. Some methodological problems concerning the kinetic analysis of non-isothermal data for therm-oxidative degradation of polymers and polymeric materials. Poly Degrad Stab. 2005;89:265–73.
Rotaru A, Goąa M. Computational thermal and kinetic analysis: complete standard procedure to evaluate the kinetic triplet from non-isothermal data. J Therm Anal Calor. 2009;2:421.
Cai J, Liu R. Weibull mixture model for modeling nonisothermal kinetics of thermally stimulated solid-state reactions: application to simulated and real kinetic conversion data. J Phys Chem B. 2007;111:10681–6.
Peters KE, Walters CC, Mankiewicz PJ. Evaluation of kinetic uncertainty in numerical models of petroleum generation. AAPG Bull. 2006;90:1–19.
Stainforth JG. Practical kinetic modeling of petroleum generation and expulsion. Mar Pet Geol. 2009;26:552–72.
Walters CC, Freund H, Kelemen SR, Peczak P, Curry DJ. Predicting oil and gas compositional yields via chemical structure-chemical yield modeling (CY-CYM): part 2—application under laboratory and geologic conditions. Org Geochem. 2007;38:306–22.
Roduit B, Folly P, Berger B, Mathieu J, Sarbach A, Andres H, Ramin M, Vogelsanger B. Evaluating SADT by advanced kinetics-based simulation approach. J Therm Anal Calor. 2008;93:153–61.
Wemhoff AP, Howard WM, Burnham AK, Nichols AL. An LX-10 kinetic model calibrated using simulations of multiple small-scale thermal safety tests. J Phys Chem A. 2008;112:9005–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burnham, A.K. Use and misuse of logistic equations for modeling chemical kinetics. J Therm Anal Calorim 127, 1107–1116 (2017). https://doi.org/10.1007/s10973-015-4879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4879-3