Abstract
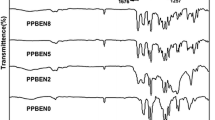
The monomer containing sulfone and amide linkages, namely N,N'-bis(4-phenoxybenzoyl)-4,4'-diaminodiphenyl sulfone (BPBDAS), was conveniently prepared by the condensation of 4,4′-diaminodiphenyl sulfone with 4-phenoxybenzoyl chloride in N,N-dimethylacetamide (DMAc). Novel copolymers of poly (ether ketone biphenyl ketone ether ketone ketone) and poly (ether ketone sulfone amide) were synthesized by the modified Friedel-Crafts acylation solution copolycondensation of isophthaloyl chloride (IPC) with a mixture of 4,4′-bis (4-phenoxybenzoyl) biphenyl (BPOBBP) and BPBDAS. The copolymers obtained were characterized by different physico-chemical techniques. The copolymers with 10–25 mol% BPBDAS are semicrystalline and had increased T gs over the conventional PEEK and PEKK due to the incorporation of rigid biphenylene moieties, sulfone and amide linkages in the main chains. The copolymers III and IV with 20–25 mol% BPBDAS had not only high T gs of 182–185 °C, but also moderate T ms of 337–339 °C, having good potential for the melt processing. The copolymers III and IV had tensile strengths of 102.2–103.5 MPa, Young’s moduli of 2.65–2.76 GPa, and elongations at break of 17.5–18.9 % and exhibited high thermal stability and excellent resistance to organic solvents.






Similar content being viewed by others
References
Rose JB (1974) Polymer 15:456–465
Ohno M, Takata T, Endo T (1995) J Polym Sci A Polym Chem 33:2647–2655
Ji XL, Yu DH, Zhang WJ, Wu ZW (1997) Polymer 38:3501–3504
Horner PJ, Whiteley RH (1991) J Mater Chem 1:271–280
He L, Chao D, Wang X, Jia X, Wang C, Liu X (2012) J Polym Res 19:9999–10006
Rose JB (1986) In High Performance Polymers: Their Origin and Development; Seymour RB, Kirshenbaum GS, Eds; Elsevier: New York, p187.
Mullins MJ, Woo EP (1987) J Macromol Sci Rev Macromol Chem Phys C 27:313–341
Staniland PA (1989) In Comprehensive Polymer Science; Allen G, Bevington JC, Eds; Pergamon Press: Oxford, Vol.5, p483
Cotter RJ (1995) Engineering Plastics: A Handbook of Polyarylethers. Gordon and Breach Science Publishers, Basel, Switzerland
Yonezawa N, Okamoto A (2009) Polym J 41:899–928
Clendinning RA, Kelsey DR, Botkin JH, Winslow PA, Youssefi M, Cotter RJ, Matzner M, Kwiatkowski GT (1993) Macromolecules 26:2361–2365
Chan KP, Wang Y, Hay AS (1995) Macromolecules 28:653–655
Colquhoun HM, Sestiaa LG, Zolotukhin MG (2000) Macromolecules 33:8907–8909
Ben-Haida A, Colquhoun HM, Hodge P, Williams DJ (2006) Macromolecules 39:6467–6472
Wang J, Gao Y, Hlil AR, Hay AS (2008) Macromolecules 41:298–300
Sakaguchi Y, Tokai M, Kato Y (1993) Polymer 34:1512–1515
Zolotukhin MG, Dosieret M, Fougnies C, Villers D, Gileva NG, Fatykhov AA (1995) Polymer 36:3575–3583
Krishnaswamy RK, Kalika DS (1996) Polymer 37:1915–1923
Zolotukhin MG, Rueda DR, Balta Calleja FJ, Cagiao ME, Sedova EA, Gileva NG (1997) Polymer 38:1471–1476
Rueda DR, Zolotukhin MG, Cagiao ME, Balta Calleja FJ, Villers D, Dosiere M (1996) Macromolecules 29:7016–7021
Zolotukhin MG, Colquhoun HM, Sestiaa LG, Rueda DR, Flot D (2003) Macromolecules 36:4766–4771
Ritter H, Thorwirth R, Muller G (1993) Makromol Chem 194:1469–1481
Ohno M, Takata T, Endo T (1994) Macromolecules 27:3447–3448
Liu B, Wang G, Hu W, Jin Y, Chen C, Jiang Z, Zhang W, Wu Z, Wei Y (2002) J Polym Sci Polym Chem Ed 40:3392–3398
Zhang Y, Sun X, Xu R, Niu Y, Wang G, Jiang Z (2006) Mater Chem Phys 99:465–469
Liu B, Hu W, Chen C, Jiang Z, Zhang W, Wu Z, Matsumoto T (2004) Polymer 45:3241–3247
Cao J, Su W, Wu Z, Kitayama T, Hatada K (1994) Polymer 35:3549–3556
Wu Z, Zheng Y, Yan H, Nakamura T, Nozawa T, Yosomiya R (1989) Angew Makromol Chem 173:163–181
Yu X, Zheng Y, Wu Z, Tang X (1990) J Appl Polym Sci 41:2649–2654
Shibata M, Cao J, Yosomiya R (1997) Polymer 38:3103–3108
Kruger KN, Zachmann HG (1993) Macromolecules 26:5202–5208
Yu Y, Xiao F, Cai M (2007) J Appl Polym Sci 104:3601–3606
Cai M, Xiao F, Ding N, Song C (2009) Polym Adv Technol 20:981–986
Yan T, Chen M, Xu Q, Cai M (2013) J Polym Res 21:336–343
Huang B, Sun Y, Wang G, Cai M (2013) J Polym Res 20:81–88
Janson V, Gors HC (1984) Preparation of aromatic polymer. PCT Int WO 8:403,891
Bonner WH (1962) Aromatic polyketones and preparation. US Patent 3065205
Acknowledgments
The authors thank the National Natural Science Foundation of China (Project 21,264,010) and the Natural Science Foundation of Jiangxi Province of China (Project 20132BAB203015) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, P., Xu, Q. et al. Synthesis and properties of novel copolymers of poly(ether ketone biphenyl ketone ether ketone ketone) and poly(ether ketone sulfone amide). J Polym Res 21, 533 (2014). https://doi.org/10.1007/s10965-014-0533-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0533-1