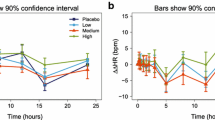
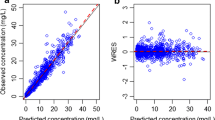
Abstract
The current method to analyze concentration-QT interval data, which is based on predictions conditional on a best model, fails to take into account the uncertainty of the model. Previous studies have suggested that failure to take into account model uncertainty using a best model approach can result in confidence intervals that are overly optimistic and may be too narrow. Theoretically, more realistic estimates are obtained using model-averaging where the overall point estimate and confidence interval are a weighted-average from a set of candidate models, the weights of which are equal to each model’s Akaike weight. Monte Carlo simulation was used to determine the degree of narrowness in the confidence interval for the degree of QT prolongation under a single ascending dose and thorough QT trial design. Results showed that model averaging performed as well as the best model approach under most conditions with no numeric advantage to using a model averaging approach. No difference was observed in the coverage of the confidence intervals when the best model and model averaging was done by AIC, AICc, or BIC, although in certain circumstances the coverage of the confidence interval themselves tended to be too narrow when using BIC. Modelers can continue to use the best model approach for concentration-QT modeling with confidence, although model averaging may offer more face validity, may be of value in cases where there is uncertainty or misspecification in the best model, and be more palatable to a non-technical reviewer than the best model approach.







Similar content being viewed by others
References
Russell T, Stein DS, Kazierad DJ (2011) Design, conduct an analysis of thorough QT studies. In: Bonate PL, Howard DR (eds) Pharmacokinetics in drug development: advances and applications. Springer, New York, pp 211–241
European Agency for the Evaluation of Medicinal Products (1997) Points to Consider: The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. https://www.fda.gov/ohrms/dockets/ac/03/briefing/pubs/cpmp.pdf. Accessed on 29 March, 2017
Britto MR, Sarapa N (2016) Clinical QTc assessment in oncology. In: Bonate PL, Howard DR (eds) Problems and challenges in oncology, vol 4. Springer International Publisher, Dordrecht, pp 77–106
Garnett CE, Beasley N, Bhattaram VA et al (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 47:13–18
United States Department of Health and Human Services, Food and Drug Administration, and Center for Drug Evaluation and Research (2005) Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (E14). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed on 29 March, 2017
Chapel S, Hutmacher MM, Bockbrader H, de Greef R, Lalonde R (2011) Comparison of QTc data analysis methods recommended by the ICH E14 guidance and exposure—response analysis: case study of a thorough QT study of asenapine. Clin Pharmacol Ther 89:75–80
Russell T, Riley SP, Cook JA, Lalonde RL (2008) A perspective on the use of concentration-QT modeling in drug development. J Clin Pharmacol 48:9–12
Tsong Y, Shen M, Zhong J, Zhang J (2008) Statistical issues of QT prolongation assessment based on linear concentration modeling. J Biopharm Stat 18:564–584
Geng J, Dang Q (2015) Simulation study for exposure-response (ER) model in QT study. Presented at Challenges and Innovations in Pharmaceutical Products Development, Durham, NC
U.S. Dept. of Health and Human Services and Food and Drug Administration (2014) Drug development and drug interactions: table of substrates, inhibitors, and inducers. https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm. Accessed 29 March, 2017
Barbour AM, Magee M, Shaddinger B et al (2015) Utility of concentration-effect modeling and simulation in a thorough QT study of losapimod. J Clin Pharmacol 55:661–670
Damle B, Fosser C, Ito K et al (2009) Effects of standard and supratherapeutic doses of nelfinavir on cardiac repolarization: a thorough QT study. J Clin Pharmacol 49:291–300
Darpo B, Karnad DR, Badilini F et al (2014) Are women more susceptible than men to drug-induced QT prolongation? Concentration-QTc modeling in a Phase 1 study with oral rac-sotalol. Br J Clin Pharmacol 77:522–531
Ferber G, Zhou M, Darpo B (2014) Detecting the QTc effect in small studies—implications for replacing the thorough QT study. Ann Noninvasive Electrocardiol 20:368–377
Darpo B, Sarapa N, Garnett CE et al (2014) The IQ-SRC prospective clinical Phase 1 study: “Can early QT assessment using exposure response analysis replace the thorough QT study?”. Ann Noninvasive Electrocardiol 19:70–81
Green JA, Patel AK, Patel BR et al (2014) Tafenoquine at therapeutic concentrations does not prolong Fridericia-corrected QT interval in healthy subjects. J Clin Pharmacol 54:995–1005
Tisdale JE, Overholser BR, Wroblenski HA et al (2012) Enhanced sensitivity to drug-induced QT interval lengthening in patients with heart failure due to left ventricular systolic dysfunction. J Clin Pharmacol 52:1296–1305
Florian JA, Tornoe CW, Brundage RC, Parekh A, Garnett CE (2011) Population pharmacokinetic and concentration–QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol 51:1152–1162
Glomb P, Ring A (2012) Delayed effects in the exposure-response analysis of clinical QTc trials. J Biopharm Stat 22:387–400
Bonate PL (2011) Pharmacokinetic—pharmacodynamic modeling and simulation, 2nd edn. Springer, New York
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Breiman L (1992) The little bootstrap and other methods for dimensionality selection in regression: X-fixed prediction error. J Am Stat Assoc 87:738–754
Bornkamp B (2015) Viewpoint: model selection uncertainty, pre-specification, and model averaging. Pharm Stat 14:79–81
Sebastien B, Hoffman D, Rigaux C, Pellisier F, Msihid J (2016) Model averaging in concentration-QT analyses. Pharm Stat 15:450–458
Jin IH, Huo L, Yin G, Yuan Y (2015) Phase I trial design for drug combinations with Bayesian model averaging. Pharm Stat 14:108–119
Schorning K, Bornkamp B, Bretz F, Dette H (2016) Model selection versus model averaging in dose finding studies. Stat Med 30:4021–4040
Verrier D, Sivapregassam S, Solente AC (2014) Dose-finding studies, MCP-Mod, model selection, and model averaging: two applications in the real world. Clin Trials 11:476–484
Pannullo F, Lee D, Waclawski E, Leyland AH (2016) How robust are the estimated effects of air pollution on health? Accounting for model uncertainty using Bayesian model averaging. Spat Spatiotemporal Epidemiol 18:53–62
Chitsazan N, Tsai F (2015) A hierarchical Bayesian model averaging framework for groundwater prediction under uncertainty. Ground Water 53:305–316
Chen JH, Chen CS, Huang MF, Lin HC (2016) Estimating the probability of rare events occurring using a local model averaging. Risk Anal 36:1855–1870
Bobb JF, Dominici F, Peng RD (2011) A, Bayesian model averaging approach for estimating the relative risk of mortality associated with heat waves in 105 U.S. cities. Biometrics 67:1605–1616
Fang X, Li R, Bottai M, Fang F, Cao Y (2016) Bayesian model averaging method for evaluating associations between air pollution and respiratory mortality: a time-series study. BMJ Open 16:e011487
Le HH, Ozer-Stillman I (2014) Use of model averaging in cost-effectiveness analysis in oncology. Value Health 17:A556
Conigliani C (2010) A Bayesian model averaging approach with non-informative priors for cost-effectiveness analyses. Stat Med 29:1696–1709
Coombes B, Basu S, Guha S, Schork N (2015) Weighted score tests implementing model-averaging schemes in detection of rare variants in case-control studies. PLoS ONE 10:e0139355
Tusell L, Perez-Rodriguez P, Forni S, Gianola D (2014) Model averaging for genome-enabled prediction with reproducing kernel Hilbert spaces: a case study with pig litter size and wheat yield. J Anim Breed Genet 131:105–115
Neto EC, Jang IS, Friend SH, Margolin AA (2014) The Stream algorithm: computationally efficient ridge-regression via Bayesian model averaging, and applications to pharmacogenomic prediction of cancer cell line sensitivity. Pac Symp Biocomput 2014:27–38
Kim H, Gelenbe E (2012) Reconstruction of large-scale gene regulatory networks using Bayesian model averaging. IEEE Trans Nanobiosci 11:259–265
Verbeke G, Molenberghs G (2000) Linear mixed models for longitudinal data. Springer, New York
Fitzmaurice GM, Laird NM, Ware JH (2004) Applied longitudinal analysis. Wiley, New York
Grosjean P, Urien S (2012) Moxifloxacin versus placebo modeling of the QT interval. J Pharmacokinet Pharmacodyn 39:205–216
Huh Y, Hutmacher M (2015) Evaluating the use of linear mixed effects models for inference of the concentration—QTc slope estimate as a surrogate for a biological QTc model. CPT 4:e00014
Bonate PL (2013) The effect of active metabolites on parameter estimation in linear mixed effects models of concentration-QT analyses. J Pharmacokinet Pharmacodyn 40:101–115
Song S, Matsushima N, Lee J, Mendell J (2015) Linear mixed-effects model of QTc prolongation for olmesartan medoxomil. J Clin Pharmacol 56:96–100
Darpo B, Garnett CE (2013) Early QT assessment—how can out confidence in the data be improved? Br J Clin Pharmacol 76:642–648
International Conference on Harmonisation and E14 Implementation Working Group (2014) ICH E14 Guideline: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs questions & answers (R2). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Q_As_R3__Step4.pdf. Accessed on 29 March, 2017
Claeskens G, Hjort NL (2010) Model selection and model averaging. Cambridge University Press, Cambridge
Garnett CE, Bonate PL, Dang Q et al (2017) Scientific white paper: best practices in concentration-QTc modeling. J Pharmacokinet Pharmacodyn (submitted)
Bloomfield DM (2015) Incorporating exposure-response modeling into the assessment of QTc interval: a potential alternative to the thorough QT study. Clin Pharmacol Ther 97:444–446
Box GEP, Draper N (1987) Empirical model building and response surfaces. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10928_2017_9523_MOESM1_ESM.png
Supplemental Figure 1 Representative concentration-time profile for one simulation under the SAD study design. Each line is a single subject (PNG 1230 kb)
10928_2017_9523_MOESM2_ESM.png
Supplemental Figure 2 Representative concentration-time profile for one simulation under the TQT study design. Each line is a single subject (PNG 893 kb)
10928_2017_9523_MOESM3_ESM.png
Supplemental Figure 3 Representative concentration-ddQTcF profiles for five simulations under a SAD study design with a circadian baseline. Solid line is the linear regression fit to the data. Blue symbols were simulations where the slope of the concentration-ddQTcF interval was statistically significant (p<0.05) using standard Model 2 (PNG 1128 kb)
10928_2017_9523_MOESM4_ESM.png
Supplemental Figure 4 Representative concentration-ddQTcF profiles for five simulations under a TQT study design with a circadian baseline. Solid line is the linear regression fit to the data. Blue symbols were simulations where the slope of the concentration-ddQTcF interval was statistically significant (p<0.05) using standard Model 2 (PNG 1854 kb)
10928_2017_9523_MOESM5_ESM.png
Supplemental Figure 5 Stacked bar chart of best model selected for each level of slope stratified by information criterion used for model selection, study design, and baseline. For the TQT design, 9 sampling points were used in the chart (PNG 830 kb)
10928_2017_9523_MOESM6_ESM.png
Supplemental Figure 6 Shape of the drug effect profile as a function of concentration for Eq. (10) to (12) stratified by slope (PNG 641 kb)
10928_2017_9523_MOESM7_ESM.png
Supplemental Figure 7 Panel plot from Monte Carlo simulation of the ratio of the best model estimate to the MA approach for the TQT study design where the data generating model was noninclusive, the baseline was circadian, and using AICc as the selection metric. The median ratio in percent of the best model estimate to the model averaged estimate plotted as a function of the slope of the true slope parameter stratified by selection criteria, different levels of Cmax (200, 500, 1000, and 2000 ng/mL), and different sampling schemes (5, 9, and 13 data points). Plotted are the mean, upper 2-sided 90% CI, and CI range. A value of 100% implies no difference between estimates. Legend: open blue circle, point estimate; dark red plus, CI range; green cross, upper confidence interval (PNG 884 kb)
10928_2017_9523_MOESM8_ESM.png
Supplemental Figure 8 Panel plot from Monte Carlo simulation of the ratio of the best model estimate to the MA approach for the TQT study design where the data generating model was noninclusive, the baseline was circadian, and using BIC as the selection metric. The median ratio in percent of the best model estimate to the model averaged estimate plotted as a function of the slope of the true slope parameter stratified by selection criteria, different levels of Cmax (200, 500, 1000, and 2000 ng/mL), and different sampling schemes (5, 9, and 13 data points). Plotted are the mean, upper 2-sided 90% CI, and CI range. A value of 100% implies no difference between estimates. Legend: open blue circle, point estimate; dark red plus, CI range; green cross, upper confidence interval (PNG 881 kb)
10928_2017_9523_MOESM9_ESM.png
Supplemental Figure 9 Stacked bar chart of best model selected for each level of slope stratified by data generating equation, study design, baseline, and information criterion used for model selection when the data generating mechanism was non-inclusive. For the TQT design, 9 sampling points and a circadian baseline were used in the chart (PNG 681 kb)
Rights and permissions
About this article
Cite this article
Bonate, P.L. Estimation of QT interval prolongation through model-averaging. J Pharmacokinet Pharmacodyn 44, 335–349 (2017). https://doi.org/10.1007/s10928-017-9523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-017-9523-3